The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus
- PMID: 9557708
- PMCID: PMC109648
- DOI: 10.1128/JVI.72.5.4192-4204.1998
The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus
Abstract
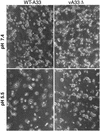
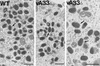
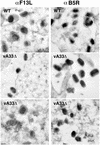
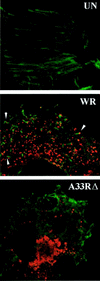
The vaccinia virus (VV) A33R gene encodes a highly conserved 23- to 28-kDa glycoprotein that is specifically incorporated into the viral outer envelope. The protein is expressed early and late after infection, consistent with putative early and late promoter sequences. To determine the role of the protein, two inducible A33R mutants were constructed, one with the late promoter and one with the early and late A33R promoter elements. Decreased A33R expression was associated with small plaques that formed comets in liquid medium. Using both an antibiotic resistance gene and a color marker, an A33R deletion mutant, vA33delta, was isolated, indicating that the A33R gene is not essential for VV replication. The plaques formed by vA33delta, however, were tiny, indicating that the A33R protein is necessary for efficient cell-to-cell spread. Rescue of the large-plaque phenotype was achieved by inserting a new copy of the A33R gene into the thymidine kinase locus, confirming the specific genetic basis of the phenotype. Although there was a reduction in intracellular virus formed in cells infected with vA33delta, the amount of infectious virus in the medium was increased. The virus particles in the medium had the buoyant density of extracellular enveloped viruses (EEV). Additionally, amounts of vA33delta cell-associated extracellular enveloped viruses (CEV) were found to be normal. Immunogold electron microscopy of cells infected with vA33delta demonstrated the presence of the expected F13L and B5R proteins in wrapping membranes and EEV; however, fully wrapped vA33delta intracellular enveloped viruses (IEV) were rare compared to partially wrapped particles. Specialized actin tails that propel IEV particles to the periphery and virus-tipped microvilli (both common in wild-type-infected cells) were absent in cells infected with vA33delta. This is the first deletion mutant in a VV envelope gene that produces at least normal amounts of fully infectious EEV and CEV and yet has a small-plaque phenotype. These data support a new model for VV spread, emphasizing the importance of virus-tipped actin tails.
Figures





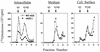

References
-
- Appleyard G, Hapel A J, Boulter E A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971;13:9–17. - PubMed
-
- Boulter E A, Appleyard G. Differences between extracellular and intracellular forms of poxvirus and their implications. Prog Med Virol. 1973;16:86–108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

