Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells
- PMID: 9557727
- PMCID: PMC109667
- DOI: 10.1128/JVI.72.5.4371-4378.1998
Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells
Abstract
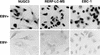
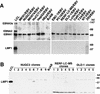
We show clear evidence for direct infection of various human epithelial cells by Epstein-Barr virus (EBV) in vitro. The successful infection was achieved by using recombinant EBV (Akata strain) carrying a selective marker gene but without any other artificial operations, such as introduction of the known EBV receptor (CD21) gene or addition of polymeric immunoglobulin A against viral gp350 in culture. Of 21 human epithelial cell lines examined, 18 became infected by EBV, as ascertained by the detection of EBV-determined nuclear antigen (EBNA) 1 expression in the early period after virus exposure, and the following selection culture easily yielded a number of EBV-infected clones from 15 cell lines. None of the human fibroblasts and five nonhuman-derived cell lines examined was susceptible to the infection. By comparison, cocultivation with virus producers showed approximately 800-fold-higher efficiency of infection than cell-free infection did, suggesting the significance of direct cell-to-cell contact as a mode of virus spread in vivo. Most of the epithelial cell lines infectable with EBV were negative for CD21 expression at the protein and mRNA levels. The majority of EBV-infected clones established from each cell line invariably expressed EBNA1, EBV-encoded small RNAs, rightward transcripts from the BamHI-A region of the virus genome, and latent membrane protein (LMP) 2A, but not the other EBNAs or LMP1. This restricted form of latent viral gene expression, which is a central issue for understanding epithelial oncogenesis by EBV, resembled that seen in EBV-associated gastric carcinoma and LMP1-negative nasopharyngeal carcinoma. The results indicate that direct infection of epithelial cells by EBV may occur naturally in vivo, and this could be mediated by an unidentified, epithelium-specific binding receptor for EBV. The EBV convertants are viewed, at least in terms of viral gene expression, as in vitro analogs of EBV-associated epithelial tumor cells, thus facilitating analysis of an oncogenic role(s) for EBV in epithelial cells.
Figures




References
-
- Aden D P, Fogel A, Plotkin S, Damjanov I, Knowles B B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979;282:615–616. - PubMed
-
- Akiyama S, Amo H, Watanabe T, Matsuyama M, Sakamoto J, Imaizumi M, Ichihashi H, Kondo T, Takagi H. Characteristics of three human gastric cancer cell lines, NU-GC-2, NU-GC-3 and NU-GC-4. Jpn J Surg. 1988;18:438–446. - PubMed
-
- Bayliss G J, Wolf H. Epstein-Barr virus-induced cell fusion. Nature. 1980;287:164–165. - PubMed
-
- Bubenik J, Baresova M, Viklicky V, Jakoubkova J, Sainerova H, Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973;11:765–773. - PubMed
-
- Cann A J, Chen I S Y. Human T-cell leukemia virus type I and II. In: Fields B N, Knipe D M, Howley P M, editors. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1849–1880.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

