Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus
- PMID: 9557737
- PMCID: PMC109677
- DOI: 10.1128/JVI.72.5.4434-4441.1998
Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus
Abstract
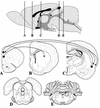
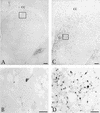
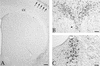
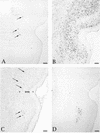
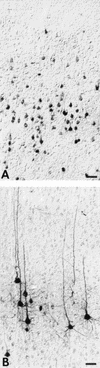
Pseudorabies virus (PRV), a swine neurotropic alphaherpesvirus, is known to invade the central nervous system (CNS) of a variety of animal species through peripherally projecting axons, replicate in the parent neurons, and then pass transsynaptically to infect other neurons of a circuit. Studies of the human pathogen herpes simplex virus type 1 have reported differences in the direction of transport of two strains of this virus after direct injection into the primate motor cortex. In the present study we examined the direction of transport of virulent and attenuated strains of PRV, utilizing injections into the rat prefrontal cortex to evaluate specific movement of virus through CNS circuitry. The data demonstrate strain-dependent patterns of infection consistent with bidirectional (anterograde and retrograde) transport of virulent virus and unidirectional (retrograde) transport of attenuated PRV from the site of injection. The distribution of infected neurons and the extent of transsynaptic passage also suggest that a release defect in the attenuated strain reduces the apparent rate of viral transport through neuronal circuitry. Finally, injection of different concentrations of virus influenced the onset of replication within a neural circuit. Taken together, these data suggest that viral envelope glycoproteins and virus concentration at the site of injection are important determinants of the rate and direction of viral transport through a multisynaptic circuit in the CNS.
Figures
References
-
- Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. - PubMed
-
- Bartha A. Experimental reduction of virulence of Aujeszky’s disease virus. Magy Allatorv Lapja. 1961;16:42–45.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

