In vitro and in vivo antifungal activity of amphotericin B lipid complex: are phospholipases important?
- PMID: 9559780
- PMCID: PMC105539
- DOI: 10.1128/AAC.42.4.767
In vitro and in vivo antifungal activity of amphotericin B lipid complex: are phospholipases important?
Abstract
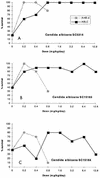
Amphotericin B lipid complex for injection (ABLC) is a suspension of amphotericin B complexed with the lipids L-alpha-dimyristoylphosphatidylcholine (DMPC) and L-alpha-dimyristoylphosphatidylglycerol. ABLC is less toxic than amphotericin B deoxycholate (AmB-d), while it maintains the antifungal activity of AmB-d. Active amphotericin B can be released from ABLC by exogenously added (snake venom, bacteria, or Candida-derived) phospholipases or by phospholipases derived from activated mammalian vascular tissue (rat arteries). Such extracellular phospholipases are capable of hydrolyzing the major lipid in ABLC. Mutants of C. albicans that were resistant to ABLC but not AmB-d in vitro were deficient in extracellular phospholipase activity, as measured on egg yolk agar or as measured by their ability to hydrolyze DMPC in ABLC. ABLC was nevertheless effective in the treatment of experimental murine infections produced by these mutants. Isolates of Aspergillus species, apparently resistant to ABLC in vitro (but susceptible to AmB-d), were also susceptible to ABLC in vivo. We suggest that routine in vitro susceptibility tests with ABLC itself as the test material may not accurately predict the in vivo activity of ABLC and that the enhanced therapeutic index of ABLC relative to that of AmB-d in vivo may be due, in part, to the selective release of active amphotericin B from the complex at sites of fungal infection through the action of fungal or host cell-derived phospholipases.
Figures


Similar articles
-
Amphotericin B formulations: a comparative review of efficacy and toxicity.Drugs. 2013 Jun;73(9):919-34. doi: 10.1007/s40265-013-0069-4. Drugs. 2013. PMID: 23729001 Review.
-
Fungal phospholipase activity and susceptibility to lipid preparations of amphotericin B.Antimicrob Agents Chemother. 2001 Nov;45(11):3231-3. doi: 10.1128/AAC.45.11.3231-3233.2001. Antimicrob Agents Chemother. 2001. PMID: 11600388 Free PMC article.
-
Comparative in vitro antifungal activity of amphotericin B lipid complex, amphotericin B and fluconazole.Chemotherapy. 2000 Jul-Aug;46(4):235-44. doi: 10.1159/000007295. Chemotherapy. 2000. PMID: 10859429
-
Behavior of amphotericin B lipid complex in plasma in vitro and in the circulation of rats.Antimicrob Agents Chemother. 1997 May;41(5):886-92. doi: 10.1128/AAC.41.5.886. Antimicrob Agents Chemother. 1997. PMID: 9145839 Free PMC article.
-
Amphotericin B lipid complex.Ann Pharmacother. 1997 Oct;31(10):1174-86. doi: 10.1177/106002809703101011. Ann Pharmacother. 1997. PMID: 9337444 Review.
Cited by
-
Amphotericin B formulations: a comparative review of efficacy and toxicity.Drugs. 2013 Jun;73(9):919-34. doi: 10.1007/s40265-013-0069-4. Drugs. 2013. PMID: 23729001 Review.
-
Potential role of phospholipases in virulence and fungal pathogenesis.Clin Microbiol Rev. 2000 Jan;13(1):122-43, table of contents. doi: 10.1128/CMR.13.1.122. Clin Microbiol Rev. 2000. PMID: 10627494 Free PMC article. Review.
-
Antifungals in systemic neonatal candidiasis.Drugs. 2004;64(9):949-68. doi: 10.2165/00003495-200464090-00003. Drugs. 2004. PMID: 15101785 Review.
-
Fungal phospholipase activity and susceptibility to lipid preparations of amphotericin B.Antimicrob Agents Chemother. 2001 Nov;45(11):3231-3. doi: 10.1128/AAC.45.11.3231-3233.2001. Antimicrob Agents Chemother. 2001. PMID: 11600388 Free PMC article.
-
Comparison of in vitro activity of liposomal nystatin against Aspergillus species with those of nystatin, amphotericin B (AB) deoxycholate, AB colloidal dispersion, liposomal AB, AB lipid complex, and itraconazole.Antimicrob Agents Chemother. 1999 May;43(5):1264-6. doi: 10.1128/AAC.43.5.1264. Antimicrob Agents Chemother. 1999. PMID: 10223948 Free PMC article.
References
-
- Calderone, R., R. Diamond, J.-M. Senet, J. Warmington, S. Filler, and J. E. Edwards. 1994. Host cell-fungal cell interactions. J. Med. Vet. Mycol. 32(Suppl. 1):151–168. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

