In vitro evaluation of drug susceptibilities of Babesia divergens isolates
- PMID: 9559789
- PMCID: PMC105548
- DOI: 10.1128/AAC.42.4.818
In vitro evaluation of drug susceptibilities of Babesia divergens isolates
Abstract
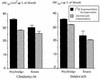
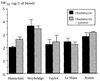
The susceptibilities of three bovine and two human Babesia divergens isolates to antimicrobial agents were evaluated in vitro by a tritiated hypoxanthine incorporation assay. The MICs at which 50% of isolates are inhibited (MIC50s) for mefloquine (chlorhydrate), chloroquine (sulfate), quinine (chlorhydrate), clindamycin (phosphate), pentamidine (isethionate), phenamidine (isethionate) plus oxomemazine (chlorhydrate), lincomycin (chlorhydrate monohydrate), and imidocarb (dipropionate) were determined. Except for imidocarb, the MIC50s observed for the different isolates were close. Imidocarb and the combination of phenamidine plus oxomemazine exhibited the highest in vitro activity, while antimalarial agents such as mefloquine, choroquine, and quinine were inactive. Other drugs had intermediate activities. The data support further in vitro evaluation of antimicrobial agents active against B. divergens for the improvement of therapeutic strategies.
Figures

References
-
- Bayle J, Dumon H, Verdot J J, Muratore R. Human piroplasmosis. One case. Presse Med. 1979;8:3674. - PubMed
-
- Beck P, Gorenflot A, Estebe J P, Marchand A, Roquilly A. Insuffisance respiratoire aigüe d’un cas de babésiose humaine. Bull Soc Fr Parasitol. 1987;5:16.
-
- Brasseur P, Lecoublet S, Kapel N, Favennec L, Ballet J J. Quinine in the treatment of Babesia divergens infections in humans. Eur J Clin Microbiol Infect Dis. 1996;15:840–841. - PubMed
-
- Brockelman C R, Tan-Ariya P. Development of an in vitro microtest to assess drug susceptibility of Babesia bovis and Babesia bigemina. J Parasitol. 1991;77:994–997. - PubMed
-
- Entrican J H, Williams H, Cook I A, Lancaster W M, Clark J C, Joyner L P. Babesiosis in man: report of a case from Scotland with observations on the infecting strain. J Infect. 1979;1:227–234.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

