Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults
- PMID: 9559795
- PMCID: PMC105554
- DOI: 10.1128/AAC.42.4.849
Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults
Abstract
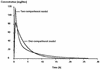
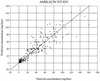
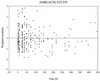
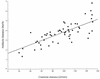
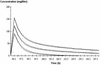
Once-daily (o.d.) administration of 20 mg of amikacin per kg of body weight to neutropenic patients has been validated by clinical studies, but amikacin pharmacokinetics have been documented only for the 7.5-mg/kg twice-daily (b.i.d.) regimen in this population. In order to determine in neutropenic patients (i) the influence of the dosing regimen on the kinetics of amikacin, (ii) the linearity of kinetics of amikacin in the range of 7.5 to 20 mg/kg, and (iii) the influence of patient characteristics on the disposition of amikacin and (iv) to provide a rationale for dosing recommendations, we evaluated the population pharmacokinetics of amikacin administered to 57 febrile neutropenic adults (neutrophil count, <500/mm3) being treated for a hematological disorder and receiving amikacin at 7.5 mg/kg b.i.d. (n = 29) or 20 mg/kg o.d. (n = 28) and administered intravenously over 0.5 h. A total of 278 blood samples were obtained (1 to 14 samples per patient) during one or several administration intervals (1 to 47). Serum amikacin levels were measured by the enzyme-multiplied immunoassay technique. A mixed-effect modeling approach was used to fit a bicompartmental model to the data (NONMEM software). The influences of the dosing regimen and the demographic and biological indices on the pharmacokinetic parameters of amikacin were evaluated by the maximum-likelihood ratio test on the population model. The dosing regimen had no influence on amikacin pharmacokinetic parameters, i.e., the kinetics of amikacin were linear over the range of 7.5 to 20 mg/kg. Amikacin elimination clearance (CL) was only correlated with creatinine clearance or its covariates, namely, sex, age, body weight, and serum creatinine level. The interindividual variability of CL was 21%, while those of the central volume of distribution, the distribution clearance, and the tissue volume of distribution were 15, 30, and 25%, respectively. On the basis of the expected distribution of amikacin concentrations in this population, dosing recommendations as a function of creatinine clearance (CL[CR]) are proposed: for patients with normal renal function (CL[CR] of 80 to 130 ml/min), 20 mg/kg o.d. is recommended, whereas for patients with severe renal impairment (CL[CR], 10 to 20 ml/min), a dosage of 17 mg/kg every 48 h is recommended.
Figures
References
-
- Beal S L, Boeckman A, Sheiner L B. NONMEM user’s guides, version IV. San Francisco: NONMEM Project Group, University of California; 1992.
-
- Beaucaire, G., O. Leroy, C. Beuscart, P. Karp, C. Chidiac, and M. Caillaux. 1991. Clinical and bacteriological efficacy, and practical aspects of amikacin given once daily for severe infections. J. Antimicrob. Chemother. 27(Suppl. C):91–103. - PubMed
-
- Bennett J E, Wakefield J C. A comparison of a Bayesian population method with two methods as implemented in commercially available software. J Pharmacokinet Biopharm. 1996;24:403–432. - PubMed
-
- Blaser J, Simmen H P, Thurnheer U, König C, Lüthy R. Nephrotoxicity, high frequency ototoxicity, efficacy and serum kinetics of once daily versus thrice daily dosing of netilmicin in patients with serious infections. J Antimicrob Chemother. 1995;36:803–814. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

