Leukotriene D4 and cystinyl-bis-glycine metabolism in membrane-bound dipeptidase-deficient mice
- PMID: 9560193
- PMCID: PMC20178
- DOI: 10.1073/pnas.95.9.4859
Leukotriene D4 and cystinyl-bis-glycine metabolism in membrane-bound dipeptidase-deficient mice
Abstract
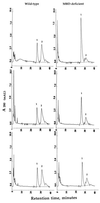
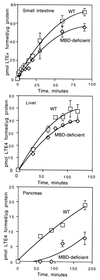
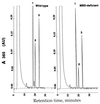
We have developed mice deficient in membrane-bound dipeptidase (MBD, EC 3.4.13.19), the enzyme believed to be responsible for the conversion of leukotriene D4 (LTD4) to leukotriene E4 (LTE4). The MBD mutation generated by us was demonstrated to be a null mutation by Northern blot analysis and the absence of beta-lactamase activity in lung, kidney, small intestine, and heart. MBD gene deletion had no effect on viability or fertility. The mutant mice retain partial ability to convert LTD4 to LTE4, ranging from 80-90% of the wild-type values in small intestine and liver to 16% in kidney and 40% in lung, heart, and pancreas. MBD is also believed to function consecutively after gamma-glutamyl transpeptidase to cleave cystinyl-bis-glycine (cys-bis-gly) generated from glutathione cleavage. Our data indicate that kidney homogenates from MBD-deficient mice retain approximately 40% of their ability to cleave cys-bis-gly, consistent with only modest elevations (3-5-fold) of cys-bis-gly in urine from MBD-deficient mice. These observations demonstrate that the conversion of LTD4 to LTE4 and the degradation of cys-bis-gly are catalyzed by at least two alternative pathways (one of which is MBD) that complement each other to varying extents in different tissues.
Figures

References
-
- Henderson W R., Jr Ann Intern Med. 1994;121:684–697. - PubMed
-
- Lewis R A, Austen K F, Soberman R J. N Engl J Med. 1990;323:645–655. - PubMed
-
- O’Byrne P M. Ann NY Acad Sci. 1994;744:251–261. - PubMed
-
- Fauler J, Neumann C, Tsikas D, Frolich J C. J Invest Dermatol. 1992;99:8–11. - PubMed
-
- Fauler J, Thon A, Tsikas D, von der Hardt H, Frolich J C. Arthritis Rheum. 1994;37:93–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

