Endogenous cellulases in animals: isolation of beta-1, 4-endoglucanase genes from two species of plant-parasitic cyst nematodes
- PMID: 9560201
- PMCID: PMC20186
- DOI: 10.1073/pnas.95.9.4906
Endogenous cellulases in animals: isolation of beta-1, 4-endoglucanase genes from two species of plant-parasitic cyst nematodes
Abstract
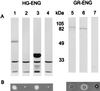
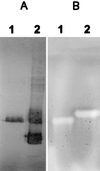
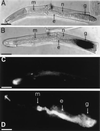
beta-1,4-Endoglucanases (EGases, EC 3.2.1.4) degrade polysaccharides possessing beta-1,4-glucan backbones such as cellulose and xyloglucan and have been found among extremely variegated taxonomic groups. Although many animal species depend on cellulose as their main energy source, most omnivores and herbivores are unable to produce EGases endogenously. So far, all previously identified EGase genes involved in the digestive system of animals originate from symbiotic microorganisms. Here we report on the synthesis of EGases in the esophageal glands of the cyst nematodes Globodera rostochiensis and Heterodera glycines. From each of the nematode species, two cDNAs were characterized and hydrophobic cluster analysis revealed that the four catalytic domains belong to family 5 of the glycosyl hydrolases (EC 3.2.1, 3.2.2, and 3.2.3). These domains show 37-44% overall amino acid identity with EGases from the bacteria Erwinia chrysanthemi, Clostridium acetobutylicum, and Bacillus subtilis. One EGase with a bacterial type of cellulose-binding domain was identified for each nematode species. The leucine-rich hydrophobic core of the signal peptide and the presence of a polyadenylated 3' end precluded the EGases from being of bacterial origin. Cyst nematodes are obligatory plant parasites and the identified EGases presumably facilitate the intracellular migration through plant roots by partial cell wall degradation.
Figures


Comment in
-
Plant parasitic nematodes: digesting a page from the microbe book.Proc Natl Acad Sci U S A. 1998 Apr 28;95(9):4789-90. doi: 10.1073/pnas.95.9.4789. Proc Natl Acad Sci U S A. 1998. PMID: 9560178 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

