ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group
- PMID: 9563981
- PMCID: PMC28530
- DOI: 10.1136/bmj.316.7141.1337
ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group
Abstract
Objective: To assess effects of intravenous streptokinase, one month of oral aspirin, or both, on long term survival after suspected acute myocardial infarction.
Design: Randomised, "2 x 2 factorial," placebo controlled trial.
Setting: 417 hospitals in 16 countries.
Subjects: 17 187 patients with suspected acute myocardial infarction randomised between March 1985 and December 1987. Follow up of vital status complete to at least 1 January 1990 for 95% of all patients and to mid-1997 for the 6213 patients in United Kingdom.
Interventions: Intravenous streptokinase (1.5 MU in 1 hour) and oral aspirin (162 mg daily for 1 month) versus matching placebos.
Main outcome measures: Mortality from all causes during up to 10 years' follow up, with subgroup analyses based on 4 year follow up.
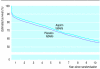
Results: After randomisation, 1841 deaths were recorded in days 0-35, 991 from day 36 to end of year 1, 1478 in years 2-4, and 1230 in years 5-10. Allocation to streptokinase was associated with 29 (95% confidence interval 20 to 38) fewer deaths per 1000 patients during days 0-35. This early benefit persisted (death rate ratio 0.98 (0.92 to 1.04) for additional deaths between day 36 and end of year 10), so that there were 28 (14 to 42) and 23 (2 to 44) fewer deaths per 1000 patients treated with streptokinase after 4 years and 10 years respectively. There was no evidence that absolute survival benefit increased with prolonged follow up among any category of patient, including those presenting early after symptoms started or with anterior ST elevation. Nor did the early benefits seem to be lost in any category (including those aged over 70). Allocation to one month of aspirin was associated with 26 (16 to 35) fewer deaths per 1000 during first 35 days, with little further benefit or loss during subsequent years (death rate ratio 0.99 (0.93 to 1.06) between day 36 and end of year 10). The early benefit obtained with combination of streptokinase and one month of aspirin also seemed to persist long term.
Conclusions: The early survival advantages produced by fibrinolytic therapy and one month of aspirin started in acute myocardial infarction seem to be maintained for at least 10 years.
Figures





References
-
- ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;ii:349–360. - PubMed
-
- ISAM (Intravenous Streptokinase in Acute Myocardial Infarction) Study Group. A prospective trial of intravenous streptokinase in acute myocardial infarction (ISAM): mortality, morbidity and infarct size at 21 days. N Engl J Med. 1986;314:1465–1471. - PubMed
-
- Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto miocardico (GISSI) Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet. 1986;i:397–402. - PubMed
-
- The AIMS (APSAC Intervention Mortality Study) Trial Study Group. Effects of intravenous APSAC on mortality after acute myocardial infarction. Preliminary report of a placebo-controlled clinical trial. Lancet. 1988;i:545–549. - PubMed
-
- Wilcox RG, von der Lippe G, Olsson CJ, Jensen G, Skene AM, Hampton JR.for the ASSET (Anglo-Scandinavian Study of Early Thrombolysis) Study Group. Trial of tissue plasminogen activator for mortality reduction in acute myocardial infarction (ASSET) Lancet 1988ii525–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
