Zoonotic filariasis
- PMID: 9564568
- PMCID: PMC106837
- DOI: 10.1128/CMR.11.2.366
Zoonotic filariasis
Abstract
Filariae of animals, especially those of mammals, often infect humans and typically produce cryptic infections. These "zoonotic" infections have been reported from virtually all parts of the world including temperate zones. Infections may be symptomatic or not, and the parasites are found in surgical tissue biopsy specimens or, more rarely, are removed intact from superficial sites such as the orbit or conjuctivae. Typically, these worms tend to occupy tissue sites similar to those occupied in the natural animal host, with the exception of the eyes. Many kinds of filariae have been isolated from humans, including species of Dirofilaria, Brugia, Onchocerca, Dipetalonema, Loaina and Meningonema. Worms have been found in subcutaneous tissues, the heart and lungs, lymphatics, the eye, and the central nervous system. Specific identification of these filariae is based on their morphological features in histologic sections. Unfortunately, some of these worms cannot be identified even at the generic level. There are other species of filariae, presumed to be zoonotic, which produce patent infections in humans but are poorly and incompletely known. These include Microfilaria semiclarum and Microfilaria bolivarensis. It is probable that almost any filaria parasitizing animals can, under proper circumstances, infect humans and undergo some degree of development. Undoubtedly, additional species of filariae will continue to be isolated from humans in the future.
Figures
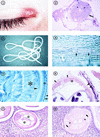


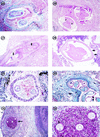
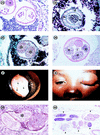

References
-
- Abadie H, Swartzwelder J C, Holman R L. A human case of Dirofilaria immitis infection. Am J Trop Med Hyg. 1965;14:117–118. - PubMed
-
- Addario C. Su un nematode dell’occhio umano. Ann Ottalmolog. 1885;13:135–147.
-
- Akashi M, Tashiro T, Got J, Nasu M, Itoga T, Tsuboi M, Araki K. A case of pulmonary dirofilariasis. Jpn J Thorac Dis. 1983;21:1228–1232. - PubMed
-
- Aldravando U. Boloniae apud Ioan Bapt. Bellagambaum; 1602. De animalibus inssectis libri septem cum singulorum iconibus ad vivum expressis; pp. 655–656.
-
- Ali-Khan Z. Tissue pathology and comparative microanatomy of Onchocerca from a resident of Ontario and other enzootic Onchocerca species from Canada and the USA. Ann Trop Med Parasitol. 1977;71:469–482. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

