Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice
- PMID: 9565645
- PMCID: PMC2212260
- DOI: 10.1084/jem.187.9.1537
Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice
Abstract
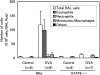
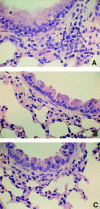
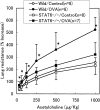
Signal transducers and activators of transcription 6 (STAT6) is essential for interleukin 4-mediated responses, including class switching to IgE and induction of type 2 T helper cells. To investigate the role of STAT6 in allergic asthma in vivo, we developed a murine model of allergen-induced airway inflammation. Repeated exposure of actively immunized C57BL/6 mice to ovalbumin (OVA) aerosol increased the level of serum IgE, the number of eosinophils in bronchoalveolar lavage (BAL) fluid, and airway reactivity. Histological analysis revealed peribronchial inflammation with pulmonary eosinophilia in OVA-treated mice. In STAT6-deficient (STAT6-/-) C57BL/6 mice treated in the same fashion, there were no eosinophilia in BAL and significantly less peribronchial inflammation than in wild-type mice. Moreover STAT6-/- mice had much less airway reactivity than wild-type mice. These findings suggest that STAT6 plays a crucial role in the pathogenesis of allergen-induced airway inflammation.
Figures

References
-
- Burrow B, Martines F, Halonen M, Barbee R, Cline M. Association of asthma with serum IgE levels and skin test reactivity to allergens. N Engl J Med. 1989;320:271–277. - PubMed
-
- Sears MR, Burrows B, Flannery EM, Herbinson GP, Hewitt CJ, Holdaway MD. Relationship between airway hyperresponsiveness and serum IgE in child with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. - PubMed
-
- Bousquet J, Chanez P, Lacote JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Lafontaine JS, Godard P, Michel FB. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. - PubMed
-
- Djukanovic R, Roche WR, Wilson JM, Beasley CRW, Twentyman OP, Howarth PH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990;141:263–275. - PubMed
-
- Gleich G. The eosinophil and bronchial asthma. Current understanding. J Allergy Clin Immunol. 1990;85:422–436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

