The Drosophila esc and E(z) proteins are direct partners in polycomb group-mediated repression
- PMID: 9566901
- PMCID: PMC110661
- DOI: 10.1128/MCB.18.5.2825
The Drosophila esc and E(z) proteins are direct partners in polycomb group-mediated repression
Abstract
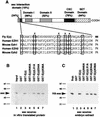
The extra sex combs (esc) and Enhancer of zeste [E(z)] proteins are members of the Drosophila Polycomb group (Pc-G) of transcriptional repressors. Here we present evidence for direct physical interaction between the esc and E(z) proteins using yeast two-hybrid and in vitro binding assays. In addition, coimmunoprecipitation from embryo extracts demonstrates association of esc and E(z) in vivo. We have delimited the esc-binding domain of E(z) to an N-terminal 33-amino-acid region. Furthermore, we demonstrate that site-directed mutations in the esc protein previously shown to impair esc function in vivo disrupt esc-E(z) interactions in vitro. We also show an in vitro interaction between the heed and EZH1 proteins, which are human homologs of esc and E(z), respectively. These results suggest that the esc-E(z) molecular partnership has been conserved in evolution. Previous studies suggested that esc is primarily involved in the early stages of Pc-G-mediated silencing during embryogenesis. However, E(z) is continuously required in order to maintain chromosome binding by other Pc-G proteins. In light of these earlier observations and the molecular data presented here, we discuss how esc-E(z) protein complexes may contribute to transcriptional silencing by the Pc-G.
Figures


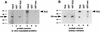
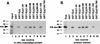


Similar articles
-
The Drosophila Polycomb Group proteins ESC and E(Z) bind directly to each other and co-localize at multiple chromosomal sites.Development. 1998 Sep;125(17):3483-96. doi: 10.1242/dev.125.17.3483. Development. 1998. PMID: 9693151
-
A Drosophila ESC-E(Z) protein complex is distinct from other polycomb group complexes and contains covalently modified ESC.Mol Cell Biol. 2000 May;20(9):3069-78. doi: 10.1128/MCB.20.9.3069-3078.2000. Mol Cell Biol. 2000. PMID: 10757791 Free PMC article.
-
Evolutionary conservation and predicted structure of the Drosophila extra sex combs repressor protein.Mol Cell Biol. 1997 Nov;17(11):6663-72. doi: 10.1128/MCB.17.11.6663. Mol Cell Biol. 1997. PMID: 9343430 Free PMC article.
-
The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3.Curr Opin Genet Dev. 2004 Apr;14(2):155-64. doi: 10.1016/j.gde.2004.02.001. Curr Opin Genet Dev. 2004. PMID: 15196462 Review.
-
An EZ mark to miss.Cell Stem Cell. 2008 Dec 4;3(6):577-8. doi: 10.1016/j.stem.2008.11.007. Cell Stem Cell. 2008. PMID: 19041770 Free PMC article. Review.
Cited by
-
Transcription factor YY1 functions as a PcG protein in vivo.EMBO J. 2003 Mar 17;22(6):1347-58. doi: 10.1093/emboj/cdg124. EMBO J. 2003. PMID: 12628927 Free PMC article.
-
The Arabidopsis thaliana genome contains at least 29 active genes encoding SET domain proteins that can be assigned to four evolutionarily conserved classes.Nucleic Acids Res. 2001 Nov 1;29(21):4319-33. doi: 10.1093/nar/29.21.4319. Nucleic Acids Res. 2001. PMID: 11691919 Free PMC article.
-
Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development.EMBO J. 2003 Sep 15;22(18):4804-14. doi: 10.1093/emboj/cdg444. EMBO J. 2003. PMID: 12970192 Free PMC article.
-
Point mutations in the WD40 domain of Eed block its interaction with Ezh2.Mol Cell Biol. 1998 Oct;18(10):5634-42. doi: 10.1128/MCB.18.10.5634. Mol Cell Biol. 1998. PMID: 9742080 Free PMC article.
-
Long-range repression by multiple polycomb group (PcG) proteins targeted by fusion to a defined DNA-binding domain in Drosophila.Genetics. 2001 May;158(1):291-307. doi: 10.1093/genetics/158.1.291. Genetics. 2001. PMID: 11333237 Free PMC article.
References
-
- Abel K J, Brody L C, Valdes J M, Erdos M R, McKinley D R, Castilla L H, Merajver S D, Couch F J, Friedman L S, Ostermeyer E A, Lynch E D, King M-C, Welcsh P L, Osborne-Lawrence S, Spillman M, Bowcock A M, Collins F S, Weber B L. Characterization of EZH1, a human homolog of Drosophila Enhancer of zeste near BRCA1. Genomics. 1996;37:161–171. - PubMed
-
- Akasaka T, Kanno M, Balling R, Antonio Mieza M, Taniguchi M, Koseki H. A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development. 1996;122:1513–1522. - PubMed
-
- Alkema M J, Bronk M, Verhoeven E, Otte A, van’t Veer L J, Berns A, van Lohuizen M. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian Polycomb complex. Genes Dev. 1997;11:226–240. - PubMed
-
- Brunk B P, Martin E C, Adler P N. Drosophila genes Posterior Sex Combs and Suppressor two of zeste encode proteins with homology to the murine bmi-1 oncogene. Nature. 1991;353:351–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
