Genetic evidence of a role for Lck in T-cell receptor function independent or downstream of ZAP-70/Syk protein tyrosine kinases
- PMID: 9566904
- PMCID: PMC110664
- DOI: 10.1128/MCB.18.5.2855
Genetic evidence of a role for Lck in T-cell receptor function independent or downstream of ZAP-70/Syk protein tyrosine kinases
Abstract
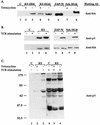
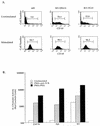
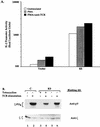
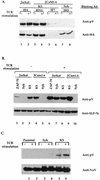
T-cell antigen receptor (TCR) engagement results in sequential activation of the Src protein tyrosine kinases (PTKs) Lck and Fyn and the Syk PTKs, ZAP-70 and Syk. While the Src PTKs mediate the phosphorylation of TCR-associated signaling subunits and the phosphorylation and activation of the Syk PTKs, the lack of a constitutively active Syk PTK has prohibited the analysis of Lck function downstream of these initiating signaling events. We describe here the generation of an activated Syk family PTK by substituting the kinase domain of Syk for the homologous region in ZAP-70 (designated as KS for kinase swap). Expression of the KS chimera resulted in its autophosphorylation, the phosphorylation of cellular proteins, the upregulation of T-cell activation markers, and the induction of interleukin-2 gene synthesis in a TCR-independent fashion. The KS chimera and downstream ZAP-70 or Syk substrates, such as SLP-76, were still phosphorylated when expressed in Lck-deficient JCaM1.6 T cells. However, expression of the KS chimera in JCaM1.6 cells failed to rescue downstream signaling events, demonstrating a functional role for Lck beyond the activation of the ZAP-70 and Syk PTKs. These results indicate that downstream TCR signaling pathways may be differentially regulated by ZAP-70 and Lck PTKs and provide a mechanism by which effector functions may be selectively activated in response to TCR stimulation.
Figures



Similar articles
-
Roles of Lck, Syk and ZAP-70 tyrosine kinases in TCR-mediated phosphorylation of the adapter protein Shc.Eur J Immunol. 1998 Aug;28(8):2265-75. doi: 10.1002/(SICI)1521-4141(199808)28:08<2265::AID-IMMU2265>3.0.CO;2-P. Eur J Immunol. 1998. PMID: 9710204
-
Reconstitution of T cell antigen receptor-induced Erk2 kinase activation in Lck-negative JCaM1 cells by Syk.Eur J Biochem. 1997 Apr 1;245(1):84-90. doi: 10.1111/j.1432-1033.1997.00084.x. Eur J Biochem. 1997. PMID: 9128727
-
Regulation of T-cell antigen receptor signalling by Syk tyrosine protein kinase.Mol Cell Biol. 1997 Aug;17(8):4434-41. doi: 10.1128/MCB.17.8.4434. Mol Cell Biol. 1997. PMID: 9234701 Free PMC article.
-
The Syk/ZAP-70 protein tyrosine kinase connection to antigen receptor signalling processes.Semin Immunol. 1995 Aug;7(4):227-36. doi: 10.1006/smim.1995.0027. Semin Immunol. 1995. PMID: 8520027 Review.
-
Interactions of TCR tyrosine based activation motifs with tyrosine kinases.Semin Immunol. 1995 Feb;7(1):13-20. doi: 10.1016/1044-5323(95)90003-9. Semin Immunol. 1995. PMID: 7612890 Review.
Cited by
-
LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation.J Exp Med. 2003 Nov 17;198(10):1463-73. doi: 10.1084/jem.20030232. Epub 2003 Nov 10. J Exp Med. 2003. PMID: 14610044 Free PMC article.
-
Mice lacking protein tyrosine kinase fyn develop a T helper-type 1 response and resistLeishmania major infection.Environ Health Prev Med. 2001 Jul;6(2):132-5. doi: 10.1007/BF02897960. Environ Health Prev Med. 2001. PMID: 21432251 Free PMC article.
-
A PLC-γ1 Feedback Pathway Regulates Lck Substrate Phosphorylation at the T-Cell Receptor and SLP-76 Complex.J Proteome Res. 2017 Aug 4;16(8):2729-2742. doi: 10.1021/acs.jproteome.6b01026. Epub 2017 Jul 6. J Proteome Res. 2017. PMID: 28644030 Free PMC article.
-
The SH3 domain of Lck modulates T-cell receptor-dependent activation of extracellular signal-regulated kinase through activation of Raf-1.Mol Cell Biol. 2008 Jan;28(2):630-41. doi: 10.1128/MCB.00150-07. Epub 2007 Nov 12. Mol Cell Biol. 2008. PMID: 17998336 Free PMC article.
-
Functional Analysis of an Inducible Promoter Driven by Activation Signals from a Chimeric Antigen Receptor.Mol Ther Oncolytics. 2018 Dec 1;12:16-25. doi: 10.1016/j.omto.2018.11.003. eCollection 2019 Mar 29. Mol Ther Oncolytics. 2018. PMID: 30662937 Free PMC article.
References
-
- Arpaia E, Shahar M, Dadi H, Cohen A, Roifman C. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking ZAP-70 kinase. Cell. 1994;76:947–958. - PubMed
-
- August A, Dupont B. Activation of src family kinase Lck following CD28 crosslinking in the Jurkat leukemic cell line. Biochem Biophys Res Commun. 1994;199:1466–1473. - PubMed
-
- Briggs S D, Bryant S S, Jove R, Sanderson S D, Smithgall T E. The ras GTPase-activating protein (GAP) is an SH3 domain-binding protein and substrate for the Src-related tyrosine kinase, Hck. J Biol Chem. 1995;270:14718–14724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
