Absence of the mid-sized neurofilament subunit decreases axonal calibers, levels of light neurofilament (NF-L), and neurofilament content
- PMID: 9566972
- PMCID: PMC2132741
- DOI: 10.1083/jcb.141.3.727
Absence of the mid-sized neurofilament subunit decreases axonal calibers, levels of light neurofilament (NF-L), and neurofilament content
Abstract
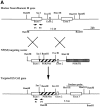
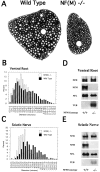
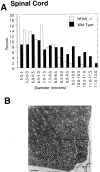
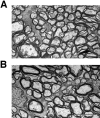
Neurofilaments (NFs) are prominent components of large myelinated axons and probably the most abundant of neuronal intermediate filament proteins. Here we show that mice with a null mutation in the mid-sized NF (NF-M) subunit have dramatically decreased levels of light NF (NF-L) and increased levels of heavy NF (NF-H). The calibers of both large and small diameter axons in the central and peripheral nervous systems are diminished. Axons of mutant animals contain fewer neurofilaments and increased numbers of microtubules. Yet the mice lack any overt behavioral phenotype or gross structural defects in the nervous system. These studies suggest that the NF-M subunit is a major regulator of the level of NF-L and that its presence is required to achieve maximal axonal diameter in all size classes of myelinated axons.
Figures










References
-
- Berthold, C.-H. 1978. Morphology of normal peripheral axons. In Physiology and Pathobiology of Axons. S.G. Waxman, editor. Raven Press, New York. 3–63.
-
- Bradley, A. 1987. Production and analysis of chimaeric mice. In Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. E.J. Robertson, editor. IRL Press, Washington, DC. 113–151.
-
- Burgoyne RD, Cambray-Deakin MA. The cellular neurobiology of neuronal development: the cerebellar granule cell. Brain Res Rev. 1988;13:77–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

