Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice
- PMID: 9570795
- PMCID: PMC6793128
- DOI: 10.1523/JNEUROSCI.18-10-03639.1998
Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice
Abstract
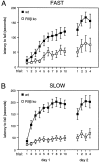
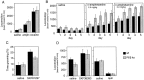
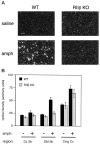
Motor behavior is modulated by dopamine-responsive neurons in the striatum, where dopaminergic signaling uses G-protein-coupled pathways, including those that result in the activation of cAMP-dependent protein kinase (PKA). The RIIbeta isoform of PKA is highly enriched in the striatum, and targeted disruption of the RIIbeta gene in mice leads to a dramatic reduction in total PKA activity in this region. Although the mutant mice show typical locomotor responses after acute administration of dopaminergic drugs, they display abnormalities in two experience-dependent locomotor behaviors: training on the rotarod task and locomotor sensitization to amphetamine. In addition, amphetamine induction of fos is absent, and the basal expression of dynorphin mRNA is reduced in the striatum. These results demonstrate that motor learning and the regulation of neuronal gene expression require RIIbeta PKA, whereas the acute locomotor effects of dopaminergic drugs are relatively unaffected by this PKA deficiency.
Figures




References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Amieux PS, Cummings DE, Motamed K, Brandon EP, Wailes LA, Le K, Idzerda RL, McKnight GS. Compensatory regulation of RIα protein levels in protein kinase A mutant mice. J Biol Chem. 1997;272:3993–3998. - PubMed
-
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Rev. 1994;19:1–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
