Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants
- PMID: 9570810
- PMCID: PMC6793168
- DOI: 10.1523/JNEUROSCI.18-10-03803.1998
Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants
Abstract
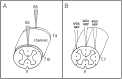
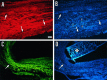
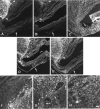
The lack of axonal regeneration in the injured adult mammalian spinal cord leads to permanent functional impairment. To induce axonal regeneration in the transected adult rat spinal cord, we have used the axonal growth-promoting properties of adult olfactory bulb ensheathing glia (EG). Schwann cell (SC)-filled guidance channels were grafted to bridge both cord stumps, and suspensions of pure (98%) Hoechst-labeled EG were stereotaxically injected into the midline of both stumps, 1 mm from the edges of the channel. In EG-transplanted animals, numerous neurofilament-, GAP-43-, anti-calcitonin gene-related peptide (CGRP)-, and serotonin-immunoreactive fibers traversed the glial scars formed at both cord-graft interfaces. Supraspinal serotonergic axons crossed the transection gap through connective tissue bridges formed on the exterior of the channels, avoiding the channel interior. Strikingly, after crossing the distal glial scar, these fibers elongated in white and periaqueductal gray matter, reaching the farthest distance analyzed (1.5 cm). Tracer-labeled axons present in SC grafts were found to extend across the distal interface and up to 800 microm beyond in the distal cord. Long-distance regeneration (at least 2.5 cm) of injured ascending propriospinal axons was observed in the rostral spinal cord. Transplanted EG migrated longitudinally and laterally from the injection sites, reaching the farthest distance analyzed (1.5 cm). They moved through white matter tracts, gray matter, and glial scars, overcoming the inhibitory nature of the CNS environment, and invaded SC and connective tissue bridges and the dorsal and ventral roots adjacent to the transection site. Transplanted EG and regenerating axons were found in the same locations. Because EG seem to provide injured spinal axons with appropriate factors for long-distance elongation, these cells offer new possibilities for treatment of CNS conditions that require axonal regeneration.
Figures





References
-
- Baird A, Klagsbrun M. The fibroblast growth factor family. Ann NY Acad Sci. 1991;638:239–243. - PubMed
-
- Baron-Van Evercooren A, Gansmuller A, Clerin E, Gumpel M. Hoechst 33342 a suitable fluorescent marker for Schwann cells after transplantation in the mouse spinal cord. Neurosci Lett. 1991;131:241–244. - PubMed
-
- Bovolenta P, Wandosell F, Nieto-Sampedro M. CNS glial scar tissue: a source of molecules which inhibit central neurite outgrowth. Prog Brain Res. 1992;94:367–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
