Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus
- PMID: 9570819
- PMCID: PMC6793142
- DOI: 10.1523/JNEUROSCI.18-10-03919.1998
Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus
Abstract
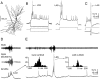
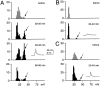
In vitro experiments suggest that dendritic fast action potentials may influence the efficacy of concurrently active synapses by enhancing Ca2+ influx into the dendrites. However, the exact circumstances leading to these effects in the intact brain are not known. We have addressed these issues by performing intracellular sharp electrode recordings from morphologically identified sites in the apical dendrites of CA1 pyramidal neurons in vivo while simultaneously monitoring extracellular population activity. The amplitude of spontaneous fast action potentials in dendrites decreased as a function of distance from the soma, suggesting that dendritic propagation of fast action potentials is strongly attenuated in vivo. Whereas the amplitude variability of somatic action potentials was very small, the amplitude of fast spikes varied substantially in distal dendrites. Large-amplitude fast spikes in dendrites occurred during population discharges of CA3-CA1 neurons concurrent with field sharp waves. The large-amplitude fast spikes were associated with bursts of smaller-amplitude action potentials and putative Ca2+ spikes. Both current pulse-evoked and spontaneously occurring Ca2+ spikes were always preceded by large-amplitude fast spikes. More spikes were observed in the dendrites during sharp waves than in the soma, suggesting that local dendritic spikes may be generated during this behaviorally relevant population pattern. Because not all dendritic spikes produce somatic action potentials, they may be functionally distinct from action potentials that signal via the axon.
Figures





References
-
- Andersen P, Bliss TVP, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Exp Brain Res. 1971;13:222–238. - PubMed
-
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Buzsáki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. - PubMed
-
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. - PubMed
-
- Buzsáki G, Leung LS, Vanderwolf CH. Cellular basis of hippocampal EEG in the behaving rat. Brain Res. 1983;6:139–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
