Sensitive periods for visual calibration of the auditory space map in the barn owl optic tectum
- PMID: 9570820
- PMCID: PMC6793138
- DOI: 10.1523/JNEUROSCI.18-10-03929.1998
Sensitive periods for visual calibration of the auditory space map in the barn owl optic tectum
Abstract
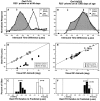
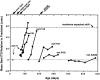
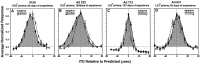
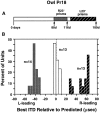
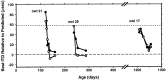
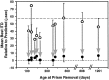
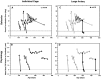
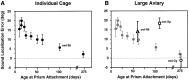
Previous studies have identified sensitive periods for the developing barn owl during which visual experience has a powerful influence on the calibration of sound localization behavior. Here we investigated neural correlates of these sensitive periods by assessing developmental changes in the capacity of visual experience to alter the map of auditory space in the optic tectum of the barn owl. We used two manipulations. (1) We equipped owls with prismatic spectacles that optically displaced the visual field by 23 degrees to the left or right, and (2) we restored normal vision to prism-reared owls that had been raised wearing prisms. In agreement with previous behavioral experiments, we found that the capacity of abnormal visual experience to shift the tectal auditory space map was restricted to an early sensitive period. However, this period extended until later in life (approximately 200 d) than described previously in behavioral studies (approximately 70 d). Furthermore, unlike the previous behavioral studies that found that the capacity to recover normal sound localization after restoration of normal vision was lost at approximately 200 d of age, we found that the capacity to recover a normal auditory space map was never lost. Finally, we were able to reconcile the behaviorally and neurophysiologically defined sensitive periods by taking into account differences in the richness of the environment in the two sets of experiments. We repeated the behavioral experiments and found that when owls were housed in a rich environment, the capacity to adjust sound localization away from normal extended to later in life, whereas the capacity to recover to normal was never lost. Conversely, when owls were housed in an impoverished environment, the capacity to recover a normal auditory space map was restricted to a period ending at approximately 200 d of age. The results demonstrate that the timing and even the existence of sensitive periods for plasticity of a neural circuit and associated behavior can depend on multiple factors, including (1) the nature of the adjustment demanded of the system and (2) the richness of the sensory and social environment in which the plasticity is studied.
Figures

References
-
- Baptista LF, Gaunt SLL. Social interaction and development in birds. In: Snowdon CT, Hausberger M, editors. Social influences on vocal development. Cambridge UP; Cambridge: 1997. pp. 23–40.
-
- Baptista L, Petrinovich L. Song development in the white-crowned sparrow: social factors and sex differences. Anim Behav. 1986;34:1359–1371.
-
- Bolhuis JJ. Mechanisms of avian imprinting: a review. Biol Rev. 1991;66:303–345. - PubMed
-
- Bottjer SW, Hewer SJ. Castration and antisteroid treatment impair vocal learning in male zebra finches. J Neurobiol. 1992;23:337–353. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
