Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala
- PMID: 9570823
- PMCID: PMC6793150
- DOI: 10.1523/JNEUROSCI.18-10-03967.1998
Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala
Abstract
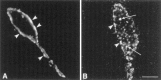
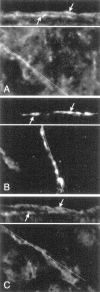
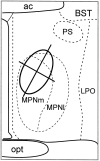
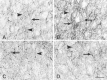
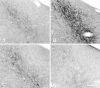
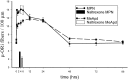
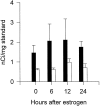
The mu-opioid receptor (mu-OR), like most G-protein-coupled receptors, is rapidly internalized after agonist binding. Although opioid peptides induce internalization in vivo, there are no studies that demonstrate mu-OR internalization in response to natural stimuli. In this study, we used laser-scanning microscopy to demonstrate that estrogen treatment induces the translocation of mu-OR immunoreactivity (mu-ORi) from the membrane to an internal location in steroid-sensitive cell groups of the limbic system and hypothalamus. Estrogen-induced internalization was prevented by the opioid antagonist naltrexone, suggesting that translocation was largely dependent on release of endogenous agonists. Estrogen treatment also altered the pattern of mu-ORi at the bright-field light microscopic level. In the absence of stimulation, the majority of immunoreactivity is diffuse, with few definable mu-OR+ cell bodies or processes. After stimulation, the density of distinct processes filled with mu-ORi was significantly increased. We interpreted the increase in the number of mu-OR+ processes as indicating increased levels of internalization. Using this increase in the density of mu-OR+ fibers, we showed that treatment of ovariectomized rats with estradiol benzoate induced a rapid and reversible increase in the number of fibers. Significant internalization was noted within 30 min and lasted for >24 hr after estrogen treatment in the medial preoptic nucleus, the principal part of the bed nucleus, and the posterodorsal medial amygdala. Naltrexone prevented the increase of mu-OR+ processes. These data imply that estrogen treatment stimulates the release of endogenous opioids that activate mu-OR in the limbic system and hypothalamus providing a "neurochemical signature" of steroid activation of these circuits.
Figures

References
-
- Allen DL, Johnson AE, Tempel A, Zukin RS, Luine VN, McEwen BS. Serotonergic lesions decrease mu- and delta-opiate receptor binding in discrete areas of the hypothalamus and in the midbrain central gray. Brain Res. 1993;625:269–275. - PubMed
-
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem. 1995;65:1636–1645. - PubMed
-
- Bhanot R, Wilkinson M. Opiatergic control of gonadotropin secretion during puberty in the rat: a neurochemical basis for the hypothalamic “gonadostat”? Endocrinology. 1983;113:596–603. - PubMed
-
- Bicknell RJ. Endogenous opioid peptides and hypothalamic neuroendocrine neurones. J Endocrinol. 1985;107:437–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
