Saccharomyces cerevisiae cells with defective spindle pole body outer plaques accomplish nuclear migration via half-bridge-organized microtubules
- PMID: 9571234
- PMCID: PMC25323
- DOI: 10.1091/mbc.9.5.977
Saccharomyces cerevisiae cells with defective spindle pole body outer plaques accomplish nuclear migration via half-bridge-organized microtubules
Abstract
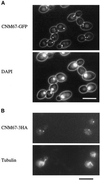
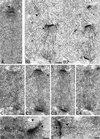
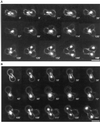
Cnm67p, a novel yeast protein, localizes to the microtubule organizing center, the spindle pole body (SPB). Deletion of CNM67 (YNL225c) frequently results in spindle misorientation and impaired nuclear migration, leading to the generation of bi- and multinucleated cells (40%). Electron microscopy indicated that CNM67 is required for proper formation of the SPB outer plaque, a structure that nucleates cytoplasmic (astral) microtubules. Interestingly, cytoplasmic microtubules that are essential for spindle orientation and nuclear migration are still present in cnm67Delta1 cells that lack a detectable outer plaque. These microtubules are attached to the SPB half- bridge throughout the cell cycle. This interaction presumably allows for low-efficiency nuclear migration and thus provides a rescue mechanism in the absence of a functional outer plaque. Although CNM67 is not strictly required for mitosis, it is essential for sporulation. Time-lapse microscopy of cnm67Delta1 cells with green fluorescent protein (GFP)-labeled nuclei indicated that CNM67 is dispensable for nuclear migration (congression) and nuclear fusion during conjugation. This is in agreement with previous data, indicating that cytoplasmic microtubules are organized by the half-bridge during mating.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

