A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation
- PMID: 9571249
- PMCID: PMC25341
- DOI: 10.1091/mbc.9.5.1195
A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation
Abstract
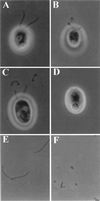
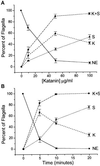
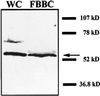
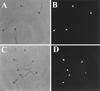
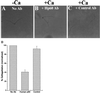
Deflagellation of Chlamydomonas reinhardtii, and other flagellated and ciliated cells, is a highly specific process that involves signal-induced severing of the outer doublet microtubules at a precise site in the transition region between the axoneme and basal body. Although the machinery of deflagellation is activated by Ca2+, the mechanism of microtubule severing is unknown. Severing of singlet microtubules has been observed in vitro to be catalyzed by katanin, a heterodimeric adenosine triphosphatase that can remove tubulin subunits from the walls of stable microtubules. We found that purified katanin induced an ATP-dependent severing of the Chlamydomonas axoneme. Using Western blot analysis and indirect immunofluorescence, we demonstrate that Chlamydomonas expresses a protein that is recognized by an anti-human katanin antibody and that this protein is localized, at least in part, to the basal body complex. Using an in vitro severing assay, we show that the protein(s) responsible for Ca2+-activated outer doublet severing purify with the flagellar-basal body complex. Furthermore, deflagellation of purified flagellar-basal body complexes is significantly blocked by the anti-katanin antibody. Taken together, these data suggest that a katanin-like mechanism may mediate the severing of the outer doublet microtubules during Chlamydomonas deflagellation.
Figures




References
-
- Blum JJ. Existence of a breaking point in cilia and flagella. J Theor Biol. 1971;33:257–263. - PubMed
-
- Dutcher SK. Purification of basal bodies and basal body complexes from Chlamydomonas reinhardtii. In: Dentler W, Witman G, editors. Cilia and Flagella. San Diego, CA: Academic Press; 1995. . Methods in Cell Biology 47, 323–334. - PubMed
-
- Dye RB, Flicker PF, Lien DY, Williams RC. End-stabilized microtubules observed in vitro: stability, subunit interchange, and breakage. Cell Motil Cytoskeleton. 1992;21:171–186. - PubMed
-
- Fabiato. A. Computer programs for calculating total from free or free from specified total ionic concentrations in aqueous solution containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. - PubMed
-
- Fechter J, Schoneberg A, Schatten G. Excision and disassembly of sperm tail microtubules during sea urchin fertilization: Requirements for microtubule dynamics. Cell Motil Cytoskeleton. 1996;35:281–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

