Evidence for kappa- and mu-opioid receptor expression in C6 glioma cells
- PMID: 9572265
- PMCID: PMC2571951
- DOI: 10.1046/j.1471-4159.1998.70051819.x
Evidence for kappa- and mu-opioid receptor expression in C6 glioma cells
Abstract
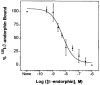
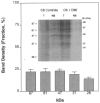
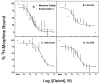
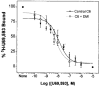
The astrocytoma cell line rat C6 glioma has been used as a model system to study the mechanism of various opioid actions. Nevertheless, the type of opioid receptor(s) involved has not been established. Here we demonstrate the presence of high-affinity U69,593, endomorphin-1, morphine, and beta-endorphin binding in desipramine (DMI)-treated C6 cell membranes by performing homologous and heterologous binding assays with [3H]U69,593, [3H]morphine, or 125I-beta-endorphin. Naive C6 cell membranes displayed U69,593 but neither endomorphin-1, morphine, nor beta-endorphin binding. Cross-linking of 125I-beta-endorphin to C6 membranes gave labeled bands characteristic of opioid receptors. Moreover, RT-PCR analysis of opioid receptor expression in control and DMI-treated C6 cells indicate that both kappa- and mu-opioid receptors are expressed. There does not appear to be a significant difference in the level of mu nor kappa receptor expression in naive versus C6 cells treated with DMI over a 20-h period. Collectively, the data indicate that kappa- and mu-opioid receptors are present in C6 glioma cells.
Figures

References
-
- Albouz S, Tocque B, Hauw JJ, Boutry JM, LeSaux F, Bourdoin R, Baumann N. Tricyclic antidepressant desipramine induces stereospecific opiate binding and lipid modification in rat glioma C6 cells. Life Sci. 1982;31:2549–2554. - PubMed
-
- Avidor-Reiss T, Nevo I, Levy R, Pfeuffer T, Vogel Z. Chronic opioid treatment induces adenylyl cyclase V superactivation. J. Biol. Chem. 1996;271:21309–21315. - PubMed
-
- Bare LA, Mansson E, Yang D. Expression of two variants of the human mu opioid receptor mRNA in SK-N-SH cells and human brain. FEBS Lett. 1994;354:213–216. - PubMed
-
- Barg J, Belcheva M, Bem WT, Lambourne B, McLachlan JA, Tolman KC, Johnson FE, Coscia CJ. Desipramine modulation of sigma and opioid peptide receptor expression in glial cells. Peptides. 1991;12:845–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

