Chaperone coexpression plasmids: differential and synergistic roles of DnaK-DnaJ-GrpE and GroEL-GroES in assisting folding of an allergen of Japanese cedar pollen, Cryj2, in Escherichia coli
- PMID: 9572938
- PMCID: PMC106217
- DOI: 10.1128/AEM.64.5.1694-1699.1998
Chaperone coexpression plasmids: differential and synergistic roles of DnaK-DnaJ-GrpE and GroEL-GroES in assisting folding of an allergen of Japanese cedar pollen, Cryj2, in Escherichia coli
Abstract
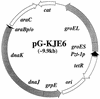
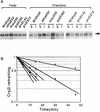
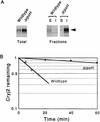
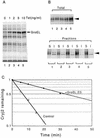
Plasmids that can be used for controlled expression of the DnaK-DnaJ-GrpE and/or GroEL-GroES chaperone team were constructed in order to facilitate assessment of the effects of these chaperone teams on folding or assembly or recombinant proteins in Escherichia coli. A typical pACYC184-based plasmid which was obtained could express the major DnaK-DnaJ-GrpE and GroEL-GroES chaperone teams from separate promoters when L-arabinose and tetracycline, respectively, were added in a dose-dependent fashion. The model protein used to determine whether this system was useful was an allergen of Japanese cedar pollen, Cryj2, which was unstable when it was produced in E. coli K-12. The effects of chaperone coexpression on the folding, aggregation, and stability of Cryj2 were examined in the wild type and in several mutant bacteria. Coexpression of the DnaK-DnaJ-GrpE and/or GroEL-GroES chaperone team at appropriate levels resulted in marked stabilization and accumulation of Cryj2 without extensive aggregation. Experiments performed with mutants that lack each of the chaperone proteins (DnaK, DnaJ, GrpE, GroEL, and GroES) or heat shock transcription factor sigma 32 revealed that both chaperone teams are critically involved in Cryj2 folding but that they are involved in distinct ways. In addition, it was observed that the two chaperone teams have synergistic roles in preventing aggregation of Cryj2 in the absence of sigma 32 at certain temperatures.
Figures


Similar articles
-
ClpB and HtpG facilitate de novo protein folding in stressed Escherichia coli cells.Mol Microbiol. 2000 Jun;36(6):1360-70. doi: 10.1046/j.1365-2958.2000.01951.x. Mol Microbiol. 2000. PMID: 10931286
-
Both the Escherichia coli chaperone systems, GroEL/GroES and DnaK/DnaJ/GrpE, can reactivate heat-treated RNA polymerase. Different mechanisms for the same activity.J Biol Chem. 1993 Dec 5;268(34):25425-31. J Biol Chem. 1993. PMID: 7902351
-
The role of DnaK/DnaJ and GroEL/GroES systems in the removal of endogenous proteins aggregated by heat-shock from Escherichia coli cells.FEBS Lett. 1999 Mar 12;446(2-3):331-7. doi: 10.1016/s0014-5793(99)00154-4. FEBS Lett. 1999. PMID: 10100869
-
Interferon-gamma is a target for binding and folding by both Escherichia coli chaperone model systems GroEL/GroES and DnaK/DnaJ/GrpE.Biochimie. 1998 Aug-Sep;80(8-9):729-37. doi: 10.1016/s0300-9084(99)80026-1. Biochimie. 1998. PMID: 9865495 Review.
-
Substrate Interaction Networks of the Escherichia coli Chaperones: Trigger Factor, DnaK and GroEL.Adv Exp Med Biol. 2015;883:271-94. doi: 10.1007/978-3-319-23603-2_15. Adv Exp Med Biol. 2015. PMID: 26621473 Review.
Cited by
-
Insights into the action of the pharmaceutical metformin: Targeted inhibition of the gut microbial enzyme agmatinase.iScience. 2024 Jan 12;27(2):108900. doi: 10.1016/j.isci.2024.108900. eCollection 2024 Feb 16. iScience. 2024. PMID: 38318350 Free PMC article.
-
Soluble Expression of Humanized Anti-CD20 Single Chain Antibody in Escherichia coli by Cytoplasmic Chaperones Co-expression.Avicenna J Med Biotechnol. 2018 Jul-Sep;10(3):141-146. Avicenna J Med Biotechnol. 2018. PMID: 30090206 Free PMC article.
-
Hfq modulates the sigmaE-mediated envelope stress response and the sigma32-mediated cytoplasmic stress response in Escherichia coli.J Bacteriol. 2007 Mar;189(5):1963-73. doi: 10.1128/JB.01243-06. Epub 2006 Dec 8. J Bacteriol. 2007. PMID: 17158661 Free PMC article.
-
Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli.Appl Environ Microbiol. 2000 Mar;66(3):884-9. doi: 10.1128/AEM.66.3.884-889.2000. Appl Environ Microbiol. 2000. PMID: 10698746 Free PMC article.
-
Genome mining unveils a class of ribosomal peptides with two amino termini.Nat Commun. 2023 Mar 23;14(1):1624. doi: 10.1038/s41467-023-37287-1. Nat Commun. 2023. PMID: 36959188 Free PMC article.
References
-
- Casadaban M. Transposition and fusion of the lac gene to selected promoters in E. coli using bacteriophage lambda and mu. J Mol Biol. 1976;104:541–555. - PubMed
-
- Georgopoulos C, Liberek K, Zylicz M, Ang D. Properties of the heat shock proteins of Escherichia coli and the autoregulation of the heat shock response. In: Morimoto R I, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 209–249.
-
- Gross C A. Function and regulation of the heat shock proteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: AMS Press; 1996. pp. 1382–1399.
-
- Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

