Grazing Pressure by a Bacterivorous Flagellate Reverses the Relative Abundance of Comamonas acidovorans PX54 and Vibrio Strain CB5 in Chemostat Cocultures
- PMID: 9572971
- PMCID: PMC106250
- DOI: 10.1128/AEM.64.5.1910-1918.1998
Grazing Pressure by a Bacterivorous Flagellate Reverses the Relative Abundance of Comamonas acidovorans PX54 and Vibrio Strain CB5 in Chemostat Cocultures
Abstract
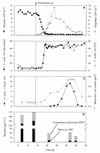
The response of the bacterial strains Comamonas acidovorans PX54 (beta subclass of the class Proteobacteria) and Vibrio strain CB5 (gamma subclass of the class Proteobacteria) to grazing by the bacterivorous flagellate Ochromonas sp. was examined in one-stage chemostat experiments under conditions of low growth rates with a complex carbon source. The two bacterial strains were cultured together; they were cultured without flagellates in the first phase of the experiments and in the presence of the flagellates in the second phase. Monoclonal and polyclonal antibodies were used to determine the numbers and sizes of C. acidovorans PX54 and Vibrio strain CB5 cells. The flagellates caused strong changes in total bacterial cell numbers, in the relative abundances of the individual bacterial strains, and in bacterial cell size distribution. Vibrio strain CB5 dominated the total bacterial cell numbers during the flagellate-free phase of the experiments with a relative abundance of 93%, but this declined to 33% after inoculation with the flagellate. In contrast to Vibrio strain CB5, C. acidovorans PX54 responded to grazing with a strong expansion of cell length distribution toward large, filamentous cells. These changes in cell morphology resulted in a high percentage of inedible cells in the C. acidovorans PX54 population but not in the Vibrio strain CB5 population, which caused the observed change in the relative abundances of the strains. Batch culture experiments without the flagellate demonstrated that the elongation of C. acidovorans PX54 cells was dependent on their growth rate. This indicates that the occurrence of filamentous C. acidovorans PX54 cells is not a direct response to chemical stimuli released by the flagellates but rather a response to increased growth rates due to flagellate grazing.
Figures







References
-
- Andersson A, Lee C, Azam F, Hagström Å. Release of amino acids and inorganic nutrients by heterotrophic marine microflagellates. Mar Ecol Prog Ser. 1985;23:99–106.
-
- Andersson A, Larsson U, Hagström Å. Size-selective grazing by a microflagellate on pelagic bacteria. Mar Ecol Prog Ser. 1986;33:51–57.
-
- Arndt H, Mathes J. Large heterotrophic flagellates form a significant part of protozooplankton biomass in lakes and rivers. Ophelia. 1991;33:225–234.
-
- Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1553–1569.
LinkOut - more resources
Full Text Sources

