Defects in regulation of apoptosis in caspase-2-deficient mice
- PMID: 9573047
- PMCID: PMC316779
- DOI: 10.1101/gad.12.9.1304
Defects in regulation of apoptosis in caspase-2-deficient mice
Abstract
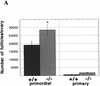
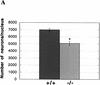
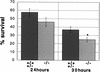
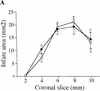
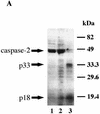
During embryonic development, a large number of cells die naturally to shape the new organism. Members of the caspase family of proteases are essential intracellular death effectors. Herein, we generated caspase-2-deficient mice to evaluate the requirement for this enzyme in various paradigms of apoptosis. Excess numbers of germ cells were endowed in ovaries of mutant mice and the oocytes were found to be resistant to cell death following exposure to chemotherapeutic drugs. Apoptosis mediated by granzyme B and perforin was defective in caspase-2-deficient B lymphoblasts. In contrast, cell death of motor neurons during development was accelerated in caspase-2-deficient mice. In addition, caspase-2-deficient sympathetic neurons underwent apoptosis more effectively than wild-type neurons when deprived of NGF. Thus, caspase-2 acts both as a positive and negative cell death effector, depending upon cell lineage and stage of development.
Figures








References
-
- Ashwell KW, Watson CRR. The development of facial motoneurons in the mouse: Neuronal death and the innervation of the facial muscle cells. J Embryol Exp Morphol. 1983;77:117–141. - PubMed
-
- Chinnaiyan AM, Dixit VM. The cell-death machine. Curr Biol. 1996;6:555–562. - PubMed
-
- Darmon AJ, Nicholson DW, Bleackley RC. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995;377:446–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
