Proteasome-independent activation of nuclear factor kappaB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii
- PMID: 9573057
- PMCID: PMC108131
- DOI: 10.1128/IAI.66.5.1827-1833.1998
Proteasome-independent activation of nuclear factor kappaB in cytoplasmic extracts from human endothelial cells by Rickettsia rickettsii
Abstract
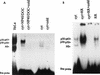
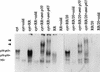
Interaction of many infectious agents with eukaryotic host cells is known to cause activation of the ubiquitous transcription factor nuclear factor kappaB (NF-kappaB) (U. Siebenlist, G. Franzoso, and K. Brown, Annu. Rev. Cell Biol. 10:405-455, 1994). Recently, we reported a biphasic pattern of NF-kappaB activation in cultured human umbilical vein endothelial cells consequent to infection with Rickettsia rickettsii, an obligate intracellular gram-negative bacterium and the etiologic agent of Rocky Mountain spotted fever (L. A. Sporn, S. K. Sahni, N. B. Lerner, V. J. Marder, D. J. Silverman, L. C. Turpin, and A. L. Schwab, Infect. Immun. 65:2786-2791, 1997). In the present study, we describe activation of NF-kappaB in a cell-free system, accomplished by addition of partially purified R. rickettsii to endothelial cell cytoplasmic extracts. This activation was rapid, reaching maximal levels at 60 min, and was dependent on the number of R. rickettsii organisms added. Antibody supershift assays using monospecific antisera against NF-kappaB subunits (p50 and p65) confirmed the authenticity of the gel-shifted complexes and identified both p50-p50 homodimers and p50-p65 heterodimers as constituents of the activated NF-kappaB pool. Activation occurred independently of the presence of endothelial cell membranes and was not inhibited by removal of the endothelial cell proteasome. Lack of involvement of the proteasome was further confirmed in assays using the peptide-aldehyde proteasome inhibitor MG 132. Activation was not ATP dependent since no change in activation resulted from addition of an excess of the unhydrolyzable ATP analog ATPgammaS, supplementation with exogenous ATP, or hydrolysis of endogenous ATP with ATPase. Furthermore, Western blot analysis before and after in vitro activation failed to demonstrate phosphorylation of serine 32 or degradation of the cytoplasmic pool of IkappaB alpha. This lack of IkappaB alpha involvement was supported by the finding that R. rickettsii can induce NF-kappaB activation in cytoplasmic extracts prepared from T24 bladder carcinoma cells and human embryo fibroblasts stably transfected with a superrepressor phosphorylation mutant of IkappaB alpha, rendering NF-kappaB inactivatable by many known signals. Thus, evidence is provided for a potentially novel NF-kappaB activation pathway wherein R. rickettsii may interact with and activate host cell transcriptional machinery independently of the involvement of the proteasome or known signal transduction pathways.
Figures





Similar articles
-
NF-kappaB activation during Rickettsia rickettsii infection of endothelial cells involves the activation of catalytic IkappaB kinases IKKalpha and IKKbeta and phosphorylation-proteolysis of the inhibitor protein IkappaBalpha.Infect Immun. 2005 Jan;73(1):155-65. doi: 10.1128/IAI.73.1.155-165.2005. Infect Immun. 2005. PMID: 15618150 Free PMC article.
-
Rickettsia rickettsii infection of cultured human endothelial cells induces NF-kappaB activation.Infect Immun. 1997 Jul;65(7):2786-91. doi: 10.1128/iai.65.7.2786-2791.1997. Infect Immun. 1997. PMID: 9199451 Free PMC article.
-
Involvement of protein kinase C in Rickettsia rickettsii-induced transcriptional activation of the host endothelial cell.Infect Immun. 1999 Dec;67(12):6418-23. doi: 10.1128/IAI.67.12.6418-6423.1999. Infect Immun. 1999. PMID: 10569758 Free PMC article.
-
Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway.Biochimie. 2001 Mar-Apr;83(3-4):351-6. doi: 10.1016/s0300-9084(01)01237-8. Biochimie. 2001. PMID: 11295496 Review.
-
Multiple redox regulation in NF-kappaB transcription factor activation.Biol Chem. 1997 Nov;378(11):1237-45. Biol Chem. 1997. PMID: 9426183 Review.
Cited by
-
New insight into immunity and immunopathology of Rickettsial diseases.Clin Dev Immunol. 2012;2012:967852. doi: 10.1155/2012/967852. Epub 2011 Sep 6. Clin Dev Immunol. 2012. PMID: 21912565 Free PMC article. Review.
-
Expression of CX3CL1 (fractalkine) in mice with endothelial-target rickettsial infection of the spotted-fever group.Virchows Arch. 2005 Jan;446(1):21-7. doi: 10.1007/s00428-004-1120-3. Epub 2004 Oct 5. Virchows Arch. 2005. PMID: 15480764
-
Host-cell interactions with pathogenic Rickettsia species.Future Microbiol. 2009 Apr;4(3):323-39. doi: 10.2217/fmb.09.6. Future Microbiol. 2009. PMID: 19327117 Free PMC article. Review.
-
Rickettsia rickettsii Whole-Cell Antigens Offer Protection against Rocky Mountain Spotted Fever in the Canine Host.Infect Immun. 2019 Jan 24;87(2):e00628-18. doi: 10.1128/IAI.00628-18. Print 2019 Feb. Infect Immun. 2019. PMID: 30396898 Free PMC article.
-
Infection of the endothelium by members of the order Rickettsiales.Thromb Haemost. 2009 Dec;102(6):1071-9. doi: 10.1160/TH09-03-0186. Thromb Haemost. 2009. PMID: 19967137 Free PMC article. Review.
References
-
- Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
-
- DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. - PubMed
-
- Goldberg A L. Functions of the proteasome: the lysis at the end of the tunnel. Science. 1995;268:522–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

