Hemoglobin-induced binding of Candida albicans to the cell-binding domain of fibronectin is independent of the Arg-Gly-Asp sequence
- PMID: 9573068
- PMCID: PMC108142
- DOI: 10.1128/IAI.66.5.1904-1909.1998
Hemoglobin-induced binding of Candida albicans to the cell-binding domain of fibronectin is independent of the Arg-Gly-Asp sequence
Abstract
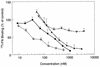
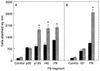
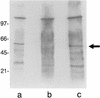
Hemoglobin specifically induces fibronectin (FN) binding to the pathogenic yeast Candida albicans. When grown in the complex medium Sabouraud broth, C. albicans expresses receptors that bind to several domains of FN. Growth in defined medium supplemented with 0.1% hemoglobin, however, enhanced the binding of FN to a single class of receptors, with a Kd = 4.6 x 10(-8) M. Competitive binding assays using recombinant and proteolytic fragments of FN revealed that the cell-binding domain mediated this interaction. A recombinant 40-kDa fragment of FN consisting of type III repeats 9 to 13 had an inhibitory activity similar to that of the entire 120-kDa cell-binding domain, indicating that the C-terminal portion of the cell-binding domain contains the binding site. A recombinant 33-kDa fragment of the cell-binding domain and a 33-kDa fragment with the RGD sequence deleted had the same inhibitory activities, demonstrating that the RGD sequence recognized by some mammalian integrins is not required. The addition of hemoglobin to the culture medium also enhanced Candida cell adhesion to immobilized FN and to 120- and 40-kDa fragments of FN but not to the collagen-binding or fibrin I domains. Using ligand protection, we identified a surface protein from C. albicans with an apparent molecular mass of 55 kDa that was protected by both FN and the 40-kDa fragment derived from the cell-binding domain. Therefore, hemoglobin both induces FN binding and changes the relative affinities of C. albicans for the cell- and collagen-binding domains of FN.
Figures

Similar articles
-
The collagen binding domain of fibronectin contains a high affinity binding site for Candida albicans.J Biol Chem. 1994 Sep 2;269(35):22039-45. J Biol Chem. 1994. PMID: 8071326
-
Binding of plasma fibronectin to Candida albicans occurs through the cell binding domain.Microb Pathog. 1994 Dec;17(6):387-93. doi: 10.1006/mpat.1994.1084. Microb Pathog. 1994. PMID: 7752880
-
Specific induction of fibronectin binding activity by hemoglobin in Candida albicans grown in defined media.Infect Immun. 1996 Aug;64(8):2930-5. doi: 10.1128/iai.64.8.2930-2935.1996. Infect Immun. 1996. PMID: 8757815 Free PMC article.
-
Hemoglobin induces binding of several extracellular matrix proteins to Candida albicans. Identification of a common receptor for fibronectin, fibrinogen, and laminin.J Biol Chem. 1998 Mar 6;273(10):5638-44. doi: 10.1074/jbc.273.10.5638. J Biol Chem. 1998. PMID: 9488693
-
Sensing the host environment: recognition of hemoglobin by the pathogenic yeast Candida albicans.Arch Biochem Biophys. 2004 Jun 15;426(2):148-56. doi: 10.1016/j.abb.2004.02.006. Arch Biochem Biophys. 2004. PMID: 15158665 Review.
Cited by
-
Candida albicans cell wall proteins.Microbiol Mol Biol Rev. 2008 Sep;72(3):495-544. doi: 10.1128/MMBR.00032-07. Microbiol Mol Biol Rev. 2008. PMID: 18772287 Free PMC article. Review.
References
-
- Aota S, Nomizu M, Yamada K M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. - PubMed
-
- Bendel C M, St. Sauver J, Carlson S, Hostetter M K. Epithelial adhesion in yeast species: correlation with surface expression of the integrin analog. J Infect Dis. 1995;171:1660–1663. - PubMed
-
- Bozzini S, Visa L, Pignatti P, Petersen T, Speziale P. Multiple binding sites in fibronectin and the staphylococcal fibronectin receptor. Eur J Biochem. 1992;207:327–333. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

