Acquired resistance of Escherichia coli to complement lysis by binding of glycophosphoinositol-anchored protectin (CD59)
- PMID: 9573071
- PMCID: PMC108145
- DOI: 10.1128/IAI.66.5.1928-1933.1998
Acquired resistance of Escherichia coli to complement lysis by binding of glycophosphoinositol-anchored protectin (CD59)
Abstract
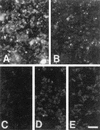
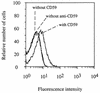
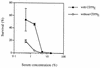
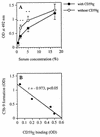
Protectin (CD59) is a glycophosphoinsitol (GPI)-anchored defender of human cells against lysis by the membrane attack complex of complement. In this study, we examined whether protectin released from human cell membranes can incorporate into the surface of gram-negative bacteria. Analysis by using radiolabeled protectin, immunofluorescence, flow cytometry, and whole-cell enzyme-linked immunosorbent assay demonstrated that protectin bound to nonencapsulated Escherichia coli EH237 (Re) and EH234 (Ra) in a calcium-dependent manner. The incorporation required the GPI-phospholipid moiety since no binding of a phospholipid-free soluble form of protectin was observed. Mg2+ did not enhance the binding, and a polysialic acid capsule prevented it (strain IH3080 [O18:K1:H8]). Bound protectin inhibited the C5b-9 neoantigen expression on complement-treated bacteria. Protection against complement lysis was observed in both a colony counting assay and a bioluminescence assay, where viable EH234 bacteria expressing the luciferase gene emitted green light in the presence of the luciferine substrate. In general, two- to four-times-higher serum concentrations were needed to obtain 50% lysis of protectin-coated versus noncoated bacteria. The results indicate that protectin can incorporate in a functionally active form into the cell membranes of the two nonencapsulated deep rough E. coli strains studied.
Figures


References
-
- Brown E J, Joiner K A, Frank M M. The role of complement in host resistance to bacteria. Springer Semin Immunopathol. 1983;6:349–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

