Integrins alpha(v)beta3 and alpha5beta1 mediate attachment of lyme disease spirochetes to human cells
- PMID: 9573074
- PMCID: PMC108148
- DOI: 10.1128/IAI.66.5.1946-1952.1998
Integrins alpha(v)beta3 and alpha5beta1 mediate attachment of lyme disease spirochetes to human cells
Abstract
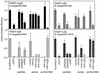
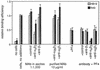
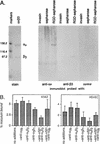
Borrelia burgdorferi (sensu lato), the agent of Lyme disease, is able to cause chronic, multisystemic infections in human and animal hosts. Attachment of the spirochete to host cells is likely to be important for the colonization of diverse tissues. The platelet-specific integrin alpha(IIb)beta3 was previously identified as a receptor for all three species of Lyme disease spirochetes (B. burgdorferi sensu stricto, B. garinii, and B. afzelii). Here we show that B. burgdorferi also recognizes the widely expressed integrins alpha(v)beta3 and alpha5beta1, known as the vitronectin and fibronectin receptors, respectively. Three representatives of each species of Lyme disease spirochete were tested for the ability to bind to purified alpha(v)beta3 and alpha5beta1. All of the strains tested bound to at least one integrin. Binding to one integrin was not always predictive of binding to other integrins, and several different integrin preference profiles were identified. Attachment of the infectious B. burgdorferi strain N40 to purified alpha(v)beta3 and alpha5beta1 was inhibited by RGD peptides and the appropriate receptor-specific antibodies. Binding to alpha(v)beta3 was also shown by using a transfected cell line that expresses this receptor but not alpha(IIb)beta3. Attachment of B. burgdorferi N40 to human erythroleukemia cells and to human saphenous vein endothelial cells was mediated by both alpha5beta1 and alpha(v)beta3. Our results show that multiple integrins mediate attachment of Lyme disease spirochetes to host cells.
Figures

References
-
- Armstrong A L, Barthold S W, Persing D H, Beck D S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992;47:249–258. - PubMed
-
- Barbour A G, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. - PubMed
-
- Barker P L, Bullens S, Bunting S, Burdick D J, Chan K S, Deisher T, Eigenbrot C, Gadek T R, Gantzos R, Lipari M T, Muir C, Napier M, Pitti R, Padus A, Quan C, Stanley M, Struble M, Tom J, Burnier J. Cyclic RGD peptide analogues as antiplatelet antithrombotics. J Med Chem. 1992;35:2040–2048. - PubMed
-
- Bodary S C, McLean J W. The integrin β1 subunit associates with the vitronectin receptor αv subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

