Binding of Porphyromonas gingivalis fimbriae to proline-rich glycoproteins in parotid saliva via a domain shared by major salivary components
- PMID: 9573091
- PMCID: PMC108165
- DOI: 10.1128/IAI.66.5.2072-2077.1998
Binding of Porphyromonas gingivalis fimbriae to proline-rich glycoproteins in parotid saliva via a domain shared by major salivary components
Abstract
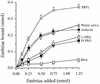
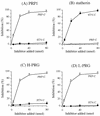
Porphyromonas gingivalis, a putative periodontopathogen, can bind to human saliva through its fimbriae. We previously found that salivary components from the submandibular and sublingual glands bind to P. gingivalis fimbriae and that acidic proline-rich protein (PRP) and statherin function as receptor molecules for fimbriae. In this study, we investigated the fimbria-binding components in parotid saliva. Fractionated human parotid saliva by gel-filtration chromatography was immobilized onto nitrocellulose membranes for the overlay assay following sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The salivary components on the membrane were allowed to interact with fimbriae purified from P. gingivalis ATCC 33277, and the interacted fimbriae were probed with anti-fimbria antibodies. The fimbriae were shown to bind to two forms of proline-rich glycoproteins (PRGs) as well as to acidic PRPs and statherin. Moreover, fimbriae bound to several components of smaller molecular size which appeared to be acidic PRP variants and basic PRPs. Fimbriae bound strongly to the purified PRGs adsorbed onto hydroxyapatite (HAP) beads. In contrast, PRGs in solution failed to inhibit the fimbrial binding to the immobilized PRGs on the HAP beads. These findings suggest that the appearance of binding site(s) of PRGs can be ascribed to their conformational changes. We previously identified the distinct segments within PRP and statherin molecules that are involved in fimbrial binding. The peptides analogous to the binding regions of PRP and statherin (i.e., PRP-C and STN-C) markedly inhibit the binding of fimbriae to PRP and statherin immobilized on the HAP beads, respectively. The PRP-C significantly inhibited the binding of fimbriae to PRG-coated HAP beads as well as to PRP on HAP beads. The peptide did not affect the binding of fimbriae to statherin, whereas the STN-C showed no effect on the fimbrial binding to PRPs or PRGs. In the overlay assay, the PRP-C clearly diminished the interactions between the fimbriae and the various salivary components, including PRPs, the PRGs, and the components with smaller molecular sizes but not statherin. These results strongly suggest that fimbriae bind to salivary components (except statherin) via common peptide segments. It is also suggested that fimbriae bind to saliva through the two distinct binding domains of receptory salivary components: (i) PRGs and PRPs and (ii) statherin.
Figures



Similar articles
-
Molecular interactions of Porphyromonas gingivalis fimbriae with host proteins: kinetic analyses based on surface plasmon resonance.Infect Immun. 1999 May;67(5):2399-405. doi: 10.1128/IAI.67.5.2399-2405.1999. Infect Immun. 1999. PMID: 10225901 Free PMC article.
-
Salivary receptors for recombinant fimbrillin of Porphyromonas gingivalis.Infect Immun. 1994 Aug;62(8):3372-80. doi: 10.1128/iai.62.8.3372-3380.1994. Infect Immun. 1994. PMID: 8039907 Free PMC article.
-
Structural domains of Porphyromonas gingivalis recombinant fimbrillin that mediate binding to salivary proline-rich protein and statherin.Infect Immun. 1996 May;64(5):1631-7. doi: 10.1128/iai.64.5.1631-1637.1996. Infect Immun. 1996. PMID: 8613371 Free PMC article.
-
Bacterial adhesion to oral tissues: a model for infectious diseases.J Dent Res. 1989 May;68(5):750-60. doi: 10.1177/00220345890680050101. J Dent Res. 1989. PMID: 2654229 Review.
-
Molecular interaction of Porphyromonas gingivalis with host cells: implication for the microbial pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):90-6. doi: 10.1902/jop.2003.74.1.90. J Periodontol. 2003. PMID: 12593602 Review.
Cited by
-
Secretion of functional salivary peptide by Streptococcus gordonii which inhibits fimbria-mediated adhesion of Porphyromonas gingivalis.Infect Immun. 1999 Aug;67(8):3780-5. doi: 10.1128/IAI.67.8.3780-3785.1999. Infect Immun. 1999. PMID: 10417138 Free PMC article.
-
Uncovering the molecular networks in periodontitis.Proteomics Clin Appl. 2014 Oct;8(9-10):748-61. doi: 10.1002/prca.201400028. Epub 2014 Jul 14. Proteomics Clin Appl. 2014. PMID: 24828325 Free PMC article. Review.
-
Molecular interactions of Porphyromonas gingivalis fimbriae with host proteins: kinetic analyses based on surface plasmon resonance.Infect Immun. 1999 May;67(5):2399-405. doi: 10.1128/IAI.67.5.2399-2405.1999. Infect Immun. 1999. PMID: 10225901 Free PMC article.
-
Group A streptococcus adheres to pharyngeal epithelial cells with salivary proline-rich proteins via GrpE chaperone protein.J Biol Chem. 2012 Jun 22;287(26):22266-75. doi: 10.1074/jbc.M112.350082. Epub 2012 May 7. J Biol Chem. 2012. PMID: 22566698 Free PMC article.
-
Characterization of the relA gene of Porphyromonas gingivalis.J Bacteriol. 2000 Jun;182(11):3302-4. doi: 10.1128/JB.182.11.3302-3304.2000. J Bacteriol. 2000. PMID: 10809717 Free PMC article.
References
-
- Amano A, Fujiwara T, Nagata H, Kuboniwa M, Sharma A, Sojar H T, Genco R J, Hamada S, Shizukuishi S. Porphyromonas gingivalis fimbriae mediate coaggregation with Streptococcus oralis through specific domains. J Dent Res. 1997;76:852–857. - PubMed
-
- Bennick A. Structural and genetic aspects of proline-rich proteins. J Dent Res. 1987;66:457–461. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

