Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants
- PMID: 9573094
- PMCID: PMC108168
- DOI: 10.1128/IAI.66.5.2093-2098.1998
Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants
Abstract
The plethora of newly discovered vaccines implies that, in the future, many vaccines will have to be administered simultaneously to infants. We examined the potential interference with the immune response of several coadministered vaccines containing the same protein component, namely, tetanus toxoid (TT). Infants simultaneously receiving a tetravalent pneumococcal vaccine conjugated to TT (PncT) and a diphtheria-tetanus-pertussis-poliovirus-Haemophilus influenzae type b-tetanus conjugate vaccine showed significantly lower anti-H. influenzae type b polysaccharide (polyribosylribitol phosphate [PRP]) antibody concentrations than those receiving either a tetravalent pneumococcal vaccine conjugated to diphtheria toxoid or placebo. A dose range study showed that anti-PRP antibody concentrations were inversely related to the TT content of the PncT vaccines administered in infancy. Postimmunization antitetanus antibody concentrations were also affected adversely as the TT content of the coadministered vaccines was increased. This phenomenon, which we believe derives from interference by a common protein carrier, should be taken into account when the introduction of an immunization program including multiple conjugate vaccines is considered.
Figures
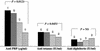
 ).
NS, not significant.
).
NS, not significant.References
-
- Ahman, H., H. Käyhty, H. Lehtonen, O. Leroy, J. Froeschle, and J. Eskola. Streptococcus pneumoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr. Infect. Dis. J., in press. - PubMed
-
- Ahman H, Käyhty H, Leroy O, Froeschle J, Eskola J. Program and Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Immunogenicity of tetravalent pneumococcal (Pnc) conjugate vaccines (PncD, PncT) in Finnish infants, abstr. G69; p. 170.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

