Generation of neutralizing antipeptide antibodies to the enzymatic domain of Pseudomonas aeruginosa exotoxin A
- PMID: 9573104
- PMCID: PMC108178
- DOI: 10.1128/IAI.66.5.2170-2179.1998
Generation of neutralizing antipeptide antibodies to the enzymatic domain of Pseudomonas aeruginosa exotoxin A
Abstract
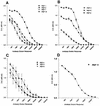
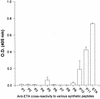
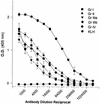
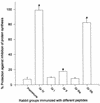
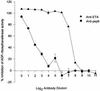
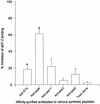
Burn patients suffer a break in the physical barrier (skin), which, when combined with their generalized state of immunodeficiency, creates an open window for opportunistic infections, mainly with Pseudomonas aeruginosa. Infection of the burn wound has always been a major factor in retardation of wound healing, and sepsis remains the leading cause of death in burn patients. Because studies have shown that topical treatment with antiexotoxin A (ETA) antibodies significantly increases survival in rats infected with toxin-producing strains of P. aeruginosa, we examined 11 synthetic peptides encompassing 12 to 45 amino acid (aa) residues, representing what were predicted by computer analysis to be the most hydrophilic and antigenic regions of ETA. These synthetic peptides were injected into rabbits for antibody production. Different groups of rabbits were immunized with a combination of peptides, with each combination representing one of the three distinct domains of ETA. Animals immunized with various peptide combinations produced peptide-specific antibodies that exhibited cross-reactivity to ETA. Two major epitopes were identified on the ETA molecule by experiments with peptide-specific antibodies in enzyme-linked immunosorbent assay and immunoprecipitation. One of these epitopes was located in the translocation domain (II) (aa 297 to 310), while the other was mapped to the last 13 aa residues at the carboxy-terminal end of the enzymatic domain (III) (aa 626 to 638). Of these two regions, the epitope in the enzymatic domain induced a much higher level of neutralizing antibodies that abrogated the cytotoxic activity of ETA in vitro. Antibodies to this epitope blocked the ADP-ribosyltransferase activity of ETA and appeared to interfere with binding of the substrate elongation factor 2 to the enzymatic active site of the ETA molecule. We conclude that polyclonal, as well as monoclonal, antibodies to short peptides, representing small regions of ETA, may have therapeutic potential in passive immunization or topical treatment of burn patients infected with toxin-producing strains of P. aeruginosa.
Figures


References
-
- Beattie B K, Prentice G A, Merrill A R. Investigation into the catalytic role for the tryptophan residues within domain III of Pseudomonas aeruginosa exotoxin A. Biochemistry. 1996;35:15134–15142. - PubMed
-
- Bryan C S, Reynolds X L, Berner E R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effect of antimicrobial therapy. Rev Infect Dis. 1983;5:629–638. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

