The surface of Toxoplasma tachyzoites is dominated by a family of glycosylphosphatidylinositol-anchored antigens related to SAG1
- PMID: 9573113
- PMCID: PMC108187
- DOI: 10.1128/IAI.66.5.2237-2244.1998
The surface of Toxoplasma tachyzoites is dominated by a family of glycosylphosphatidylinositol-anchored antigens related to SAG1
Abstract
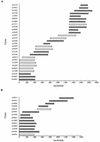
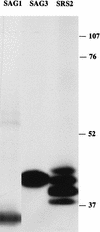
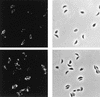
Toxoplasma gondii is an Apicomplexan parasite with a complex life cycle that includes a rapidly dividing asexual stage known as the tachyzoite. The tachyzoite surface has been reported to comprise five major antigens, the most abundant of which is designated SAG1 (for surface antigen 1). At least one of the other four (SAG3) and another recently described minor antigen (SRS1 [for SAG1-related sequence 1]) have previously been shown to be structurally related to SAG1. To determine if further SAG1 homologs exist, we searched a Toxoplasma expressed sequence tag (EST) database and found numerous ESTs corresponding to at least three new genes related to SAG1. Like SAG1, these new SRS genes encode apparently glycosylphosphatidylinositol-anchored proteins that share several motifs and a set of conserved cysteine residues. This family appears to have arisen by divergence from a common ancestor under selection for the conservation of overall topology. The products of two of these new genes (SRS2 and SRS3) are shown to be expressed on the surface of Toxoplasma tachyzoites by immunofluorescence. We also identified strain-specific differences in relative expression levels. A total of 10 members of the SAG1 gene family have now been identified, which apparently include three of the five major surface antigens previously described and one antigen expressed only in bradyzoites. The function of this family may be to provide a redundant system of receptors for interaction with host cells and/or to direct the immune responses that limit acute T. gondii infections.
Figures


Similar articles
-
Identification and characterization of SRS1, a Toxoplasma gondii surface antigen upstream of and related to SAG1.Mol Biochem Parasitol. 1997 Nov;89(2):271-82. doi: 10.1016/s0166-6851(97)00126-6. Mol Biochem Parasitol. 1997. PMID: 9364971
-
Epitopic analysis of the Toxoplasma gondii major surface antigen SAG1.Mol Biochem Parasitol. 1994 Jul;66(1):31-8. doi: 10.1016/0166-6851(94)90033-7. Mol Biochem Parasitol. 1994. PMID: 7527124
-
The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii.Infect Immun. 1998 Nov;66(11):5322-8. doi: 10.1128/IAI.66.11.5322-5328.1998. Infect Immun. 1998. PMID: 9784539 Free PMC article.
-
Toxoplasma gondii surface antigen 1 (SAG1) as a potential candidate to develop vaccine against toxoplasmosis: A systematic review.Comp Immunol Microbiol Infect Dis. 2020 Apr;69:101414. doi: 10.1016/j.cimid.2020.101414. Epub 2020 Jan 7. Comp Immunol Microbiol Infect Dis. 2020. PMID: 31958746
-
The surface of Toxoplasma: more and less.Int J Parasitol. 1998 Jan;28(1):3-9. doi: 10.1016/s0020-7519(97)00182-3. Int J Parasitol. 1998. PMID: 9504330 Review.
Cited by
-
Genetic approaches to studying virulence and pathogenesis in Toxoplasma gondii.Philos Trans R Soc Lond B Biol Sci. 2002 Jan 29;357(1417):81-8. doi: 10.1098/rstb.2001.1017. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 11839185 Free PMC article.
-
The differential protein expression profiles and immunogenicity of tachyzoites and bradyzoites of in vitro cultured Neospora caninum.Parasitol Res. 2008 Sep;103(4):905-13. doi: 10.1007/s00436-008-1075-4. Epub 2008 Jul 3. Parasitol Res. 2008. PMID: 18597117
-
Development and evaluation of an enzyme-linked immunosorbent assay based on recombinant TgSRS2 for serodiagnosis of Toxoplasma gondii infection in cats.J Vet Med Sci. 2020 Dec 5;82(11):1662-1665. doi: 10.1292/jvms.20-0231. Epub 2020 Oct 19. J Vet Med Sci. 2020. PMID: 33071252 Free PMC article.
-
Integrated bioinformatic and targeted deletion analyses of the SRS gene superfamily identify SRS29C as a negative regulator of Toxoplasma virulence.mBio. 2012 Nov 13;3(6):e00321-12. doi: 10.1128/mBio.00321-12. mBio. 2012. PMID: 23149485 Free PMC article.
-
Single Cell Expression Systems for the Production of Recombinant Proteins for Immunodiagnosis and Immunoprophylaxis of Toxoplasmosis.Microorganisms. 2024 Aug 22;12(8):1731. doi: 10.3390/microorganisms12081731. Microorganisms. 2024. PMID: 39203573 Free PMC article. Review.
References
-
- Ajioka J, Boothroyd J C, Brunk B P, Hehl A, Hillier L, Manger I D, Overton G C, Marra M, Roos D, Wan K L, Waterston R, Sibley L D. Sequencing of ESTs from the protozoan parasite Toxoplasma gondii: efficient identification of genes and identification of phylogenetically restricted sequences of the Apicomplexa. Genome Res. 1998;8:18–28. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. 1 to 3. New York, N.Y: John Wiley and Sons, Inc.; 1995.
-
- Bulow R, Boothroyd J C. Protection of mice from fatal Toxoplasma gondii infection by immunization with P30 antigen in liposomes. J Immunol. 1991;147:3496–3500. - PubMed
-
- Burg J L, Perelman D, Kasper L H, Ware P L, Boothroyd J C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. - PubMed
-
- Cesbron-Delauw M F, Tomavo S, Beauchamps P, Fourmaux M P, Camus D, Capron A, Dubremetz J F. Similarities between the primary structures of two distinct major surface proteins of Toxoplasma gondii. J Biol Chem. 1994;269:16217–16222. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

