Differential early interactions between Salmonella enterica serovar Typhi and two other pathogenic Salmonella serovars with intestinal epithelial cells
- PMID: 9573122
- PMCID: PMC108196
- DOI: 10.1128/IAI.66.5.2310-2318.1998
Differential early interactions between Salmonella enterica serovar Typhi and two other pathogenic Salmonella serovars with intestinal epithelial cells
Abstract
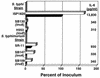
Salmonella enterica serovar Typhi (hereafter referred to as S. typhi) is a host-restricted pathogen that adheres to and invades the distal ileum and subsequently disseminates to cause typhoid fever in humans. However, S. typhi appears to be avirulent in small animals. In contrast, other pathogenic salmonellae, such as S. enterica serovars Typhimurium and Dublin (S. typhimurium and S. dublin, respectively), typically cause localized gastroenteritis in humans but have been used as models for typhoid fever because these organisms cause a disease in susceptible rodents that resembles human typhoid. In vivo, S. typhi has been demonstrated to attach to and invade murine M cells but is rapidly cleared from the Peyer's patches without destruction of the M cells. In contrast, invasion of M cells by S. typhimurium is accompanied by destruction of these M cells and subsequently sloughing of the epithelium. These data have furthered our view that the early steps in the pathogenesis of typhoidal and nontyphoidal Salmonella serovars are distinct. To extend this concept, we have utilized an in vitro model to evaluate three parameters of initial host-pathogen interactions: adherence of three Salmonella serovars to human and murine small intestinal epithelial cell (IEC) lines, the capacity of these salmonellae to invade IECs, and the ability of the bacteria to induce interleukin-6 (IL-6) in these cell lines as a measure of host cell activation and the host acute-phase response. The results demonstrate that S. typhi adheres to and invades human small IECs better than either S. typhimurium or S. dublin. Interestingly, invA and invE null mutants of S. typhi are able neither to adhere to nor to invade IECs, unlike S. typhimurium invA and invE mutants, which adhere to but cannot invade IECs. S. typhi also induces significantly greater quantities of IL-6 in human small IEC lines than either of the other two Salmonella serovars. These findings suggest that differential host cytokine responses to bacterial pathogens may play an important role in the pathological sequelae that follow infection. Importantly, S. typhimurium did not induce IL-6 in murine IECs. Since S. typhimurium infection in mice is often used as a model of typhoid fever, these findings suggest that, at least in this case, the mouse model does not reflect the human disease. Taken together, our studies indicate that (i) marked differences occur in the initial steps of S. typhi, S. typhimurium, and S. dublin pathogenesis, and (ii) conclusions about S. typhi pathogenesis that have been drawn from the mouse model of typhoid fever should be interpreted conservatively.
Figures




References
-
- Clark M A, Jepson M A, Simmons N L, Hirst B H. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res Microbiol. 1994;145:543–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

