The L-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia coli subjected to secondary environmental stresses
- PMID: 9573145
- PMCID: PMC107212
- DOI: 10.1128/JB.180.10.2623-2629.1998
The L-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia coli subjected to secondary environmental stresses
Abstract
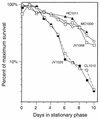
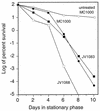
Like its homologs throughout the biological world, the L-isoaspartyl protein repair methyltransferase of Escherichia coli, encoded by the pcm gene, can convert abnormal L-isoaspartyl residues in proteins (which form spontaneously from asparaginyl or aspartyl residues) to normal aspartyl residues. Mutations in pcm were reported to greatly reduce survival in stationary phase and when cells were subjected to heat or osmotic stresses (C. Li and S. Clarke, Proc. Natl. Acad. Sci. USA 89:9885-9889, 1992). However, we subsequently demonstrated that those strains had a secondary mutation in rpoS, which encodes a stationary-phase-specific sigma factor (J. E. Visick and S. Clarke, J. Bacteriol. 179:4158-4163, 1997). We now show that the rpoS mutation, resulting in a 90% decrease in HPII catalase activity, can account for the previously observed phenotypes. We further demonstrate that a new pcm mutant lacks these phenotypes. Interestingly, the newly constructed pcm mutant, when maintained in stationary phase for extended periods, is susceptible to environmental stresses, including exposure to methanol, oxygen radical generation by paraquat, high salt concentrations, and repeated heating to 42 degrees C. The pcm mutation also results in a competitive disadvantage in stationary-phase cells. All of these phenotypes can be complemented by a functional pcm gene integrated elsewhere in the chromosome. These data suggest that protein denaturation and isoaspartyl formation may act synergistically to the detriment of aging E. coli and that the repair methyltransferase can play a role in limiting the accumulation of the potentially disruptive isoaspartyl residues in vivo.
Figures



Similar articles
-
Mutations in the Escherichia coli surE gene increase isoaspartyl accumulation in a strain lacking the pcm repair methyltransferase but suppress stress-survival phenotypes.FEMS Microbiol Lett. 1998 Oct 1;167(1):19-25. doi: 10.1111/j.1574-6968.1998.tb13202.x. FEMS Microbiol Lett. 1998. PMID: 9785447
-
Recovery from long-term stationary phase and stress survival in Escherichia coli require the L-isoaspartyl protein carboxyl methyltransferase at alkaline pH.Microbiology (Reading). 2005 Jul;151(Pt 7):2151-2158. doi: 10.1099/mic.0.27835-0. Microbiology (Reading). 2005. PMID: 16000706
-
A protein methyltransferase specific for altered aspartyl residues is important in Escherichia coli stationary-phase survival and heat-shock resistance.Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9885-9. doi: 10.1073/pnas.89.20.9885. Proc Natl Acad Sci U S A. 1992. PMID: 1409717 Free PMC article.
-
Damaged proteins bearing L-isoaspartyl residues and aging: a dynamic equilibrium between generation of isomerized forms and repair by PIMT.Curr Aging Sci. 2011 Feb;4(1):8-18. Curr Aging Sci. 2011. PMID: 21204776 Review.
-
Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair.Ageing Res Rev. 2003 Jul;2(3):263-85. doi: 10.1016/s1568-1637(03)00011-4. Ageing Res Rev. 2003. PMID: 12726775 Review.
Cited by
-
Large-scale QM/MM free energy simulations of enzyme catalysis reveal the influence of charge transfer.Phys Chem Chem Phys. 2018 Aug 8;20(31):20650-20660. doi: 10.1039/c8cp03871f. Phys Chem Chem Phys. 2018. PMID: 30059109 Free PMC article.
-
Protein-L-Isoaspartyl Methyltransferase (PIMT) Is Required for Survival of Salmonella Typhimurium at 42°C and Contributes to the Virulence in Poultry.Front Microbiol. 2017 Mar 7;8:361. doi: 10.3389/fmicb.2017.00361. eCollection 2017. Front Microbiol. 2017. PMID: 28326072 Free PMC article.
-
Extension of the Drosophila lifespan by overexpression of a protein repair methyltransferase.Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):14814-8. doi: 10.1073/pnas.251446498. Epub 2001 Dec 11. Proc Natl Acad Sci U S A. 2001. PMID: 11742076 Free PMC article.
-
Linkage map of Escherichia coli K-12, edition 10: the traditional map.Microbiol Mol Biol Rev. 1998 Sep;62(3):814-984. doi: 10.1128/MMBR.62.3.814-984.1998. Microbiol Mol Biol Rev. 1998. PMID: 9729611 Free PMC article. Review.
-
Structural investigation of a phosphorylation-catalyzed, isoaspartate-free, protein succinimide: crystallographic structure of post-succinimide His15Asp histidine-containing protein.Biochemistry. 2008 Sep 9;47(36):9486-96. doi: 10.1021/bi800847a. Epub 2008 Aug 15. Biochemistry. 2008. PMID: 18702519 Free PMC article.
References
-
- Casadaban M J, Cohen S N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980;138:179–207. - PubMed
-
- Clark D P, Beard J P. Altered phospholipid composition in mutants of Escherichia coli sensitive or resistant to organic solvents. J Gen Microbiol. 1979;113:267–274. - PubMed
-
- Clarke S. Propensity for spontaneous succinimide formation from aspartyl and asparaginyl residues in cellular proteins. Int J Pept Protein Res. 1987;30:808–821. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

