Function of protonatable residues in the flagellar motor of Escherichia coli: a critical role for Asp 32 of MotB
- PMID: 9573160
- PMCID: PMC107227
- DOI: 10.1128/JB.180.10.2729-2735.1998
Function of protonatable residues in the flagellar motor of Escherichia coli: a critical role for Asp 32 of MotB
Abstract
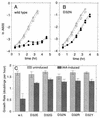
Rotation of the bacterial flagellar motor is powered by a transmembrane gradient of protons or, in some species, sodium ions. The molecular mechanism of coupling between ion flow and motor rotation is not understood. The proteins most closely involved in motor rotation are MotA, MotB, and FliG. MotA and MotB are transmembrane proteins that function in transmembrane proton conduction and that are believed to form the stator. FliG is a soluble protein located on the cytoplasmic face of the rotor. Two other proteins, FliM and FliN, are known to bind to FliG and have also been suggested to be involved to some extent in torque generation. Proton (or sodium)-binding sites in the motor are likely to be important to its function and might be formed from the side chains of acidic residues. To investigate the role of acidic residues in the function of the flagellar motor, we mutated each of the conserved acidic residues in the five proteins that have been suggested to be involved in torque generation and measured the effects on motility. None of the conserved acidic residues of MotA, FliG, FliM, or FliN proved essential for torque generation. An acidic residue at position 32 of MotB did prove essential. Of 15 different substitutions studied at this position, only the conservative-replacement D32E mutant retained any function. Previous studies, together with additional data presented here, indicate that the proteins involved in motor rotation do not contain any conserved basic residues that are critical for motor rotation per se. We propose that Asp 32 of MotB functions as a proton-binding site in the bacterial flagellar motor and that no other conserved, protonatable residues function in this capacity.
Figures
References
-
- Blair D F. How bacteria sense and swim. Annu Rev Microbiol. 1995;49:489–522. - PubMed
-
- Blair D F, Berg H C. Restoration of torque in defective flagellar motors. Science. 1988;242:1678–1681. - PubMed
-
- Blair D F, Berg H C. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell. 1990;60:439–449. - PubMed
-
- Blair D F, Berg H C. Mutations in the MotA protein of Escherichia coli reveal domains critical for proton conduction. J Mol Biol. 1991;221:1433–1442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

