Ligand induction of retinoic acid receptors alters an acute infection by murine cytomegalovirus
- PMID: 9573222
- PMCID: PMC109973
- DOI: 10.1128/JVI.72.6.4589-4600.1998
Ligand induction of retinoic acid receptors alters an acute infection by murine cytomegalovirus
Abstract
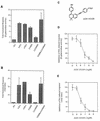
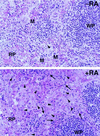
Here we report that administration of retinoids can alter the outcome of an acute murine cytomegalovirus (MCMV) infection. We show that a crucial viral control element, the major immediate-early enhancer, can be activated by retinoic acid (RA) via multiple RA-responsive elements (DR2) that bind retinoid X receptor-retinoic acid receptor (RAR) heterodimers with apparent dissociation constants ranging from 15 to 33 nM. Viral growth is dramatically increased upon RA treatment of infected tissue culture cells. Using synthetic retinoid receptor-specific agonists and antagonists, we provide evidence that RAR activation in cells is required for mediating the response of MCMV to RA. Oral administration of RA to infected immunocompetent mice selectively exacerbates an infection by MCMV, while cotreatment with an RAR antagonist protects against the adverse effects of RA on MCMV infection. In conclusion, these chemical genetic experiments provide evidence that an RAR-mediated pathway can modulate in vitro and in vivo infections by MCMV.
Figures







Similar articles
-
Characterization of the sequences of the human cytomegalovirus enhancer that mediate differential regulation by natural and synthetic retinoids.Mol Endocrinol. 1996 Jul;10(7):781-93. doi: 10.1210/mend.10.7.8813719. Mol Endocrinol. 1996. PMID: 8813719
-
The prevention of adipose differentiation of 3T3-L1 cells caused by retinoic acid is elicited through retinoic acid receptor alpha.Life Sci. 1994;55(16):PL307-12. doi: 10.1016/0024-3205(94)90073-6. Life Sci. 1994. PMID: 7934625
-
Regulation of retinoid-induced differentiation in embryonal carcinoma PCC4.aza1R cells: effects of retinoid-receptor selective ligands.Cell Growth Differ. 1996 Mar;7(3):327-37. Cell Growth Differ. 1996. PMID: 8838863
-
[Genetic control of the development by retinoic acid].C R Seances Soc Biol Fil. 1997;191(1):77-90. C R Seances Soc Biol Fil. 1997. PMID: 9181129 Review. French.
-
The retinoid receptors.Leukemia. 1994;8 Suppl 3:S1-10. Leukemia. 1994. PMID: 7808017 Review.
Cited by
-
A temporal gate for viral enhancers to co-opt Toll-like-receptor transcriptional activation pathways upon acute infection.PLoS Pathog. 2015 Apr 9;11(4):e1004737. doi: 10.1371/journal.ppat.1004737. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25856589 Free PMC article.
-
Principles of homeostasis in governing virus activation and latency.Immunol Res. 2000;21(2-3):219-23. doi: 10.1385/IR:21:2-3:219. Immunol Res. 2000. PMID: 10852120 Review.
-
Regulation of the MIE Locus During HCMV Latency and Reactivation.Pathogens. 2020 Oct 23;9(11):869. doi: 10.3390/pathogens9110869. Pathogens. 2020. PMID: 33113934 Free PMC article. Review.
-
Differentiation-Coupled Induction of Human Cytomegalovirus Replication by Union of the Major Enhancer Retinoic Acid, Cyclic AMP, and NF-κB Response Elements.J Virol. 2015 Dec;89(24):12284-98. doi: 10.1128/JVI.00965-15. Epub 2015 Sep 30. J Virol. 2015. PMID: 26423948 Free PMC article.
-
Analysis of the role of thrombomodulin in all-trans retinoic acid treatment of coagulation disorders in cancer patients.Theor Biol Med Model. 2019 Feb 14;16(1):3. doi: 10.1186/s12976-019-0099-z. Theor Biol Med Model. 2019. PMID: 30764845 Free PMC article.
References
-
- Achkar C C, Bentel J M, Boylan J F, Scher H I, Gudas L J, Miller W H J. Differences in the pharmacokinetic properties of orally administered all-trans-retinoic acid and 9-cis-retinoic acid in the plasma of nude mice. Drug Metab Dispos. 1994;22:451–458. - PubMed
-
- Agarwal C, Chandraratna R A S, Johnson A T, Rorke E A, Eckert R L. AGN 193109 is a highly effective antagonist of retinoid action in human ectocervical epithelial cells. J Biol Chem. 1996;271:12209–12212. - PubMed
-
- Angulo A, Ghazal P. Regulation of human cytomegalovirus by retinoic acid. Scand J Infect Dis. 1995;99:113–115. - PubMed
-
- Angulo, A., and P. Ghazal. Unpublished data.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

