Rescue and autonomous replication of adeno-associated virus type 2 genomes containing Rep-binding site mutations in the viral p5 promoter
- PMID: 9573246
- PMCID: PMC110022
- DOI: 10.1128/JVI.72.6.4811-4818.1998
Rescue and autonomous replication of adeno-associated virus type 2 genomes containing Rep-binding site mutations in the viral p5 promoter
Abstract
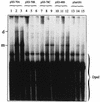
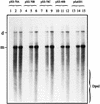
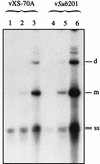
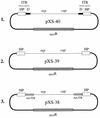
The Rep proteins encoded by the adeno-associated virus type 2 (AAV) play a crucial role in the rescue, replication, and integration of the viral genome. In the absence of a helper virus, little expression of the AAV Rep proteins occurs, and the AAV genome fails to undergo DNA replication. Since previous studies have established that expression of the Rep78 and Rep68 proteins from the viral p5 promoter is controlled by the Rep-binding site (RBS) and the YY1 factor-binding site (YBS), we constructed a number of recombinant AAV plasmids containing mutations and/or deletions of the RBS and the YBS in the p5 promoter. These plasmids were transfected in HeLa or 293 cells and analyzed for the potential to undergo AAV DNA rescue and replication. Our studies revealed that (i) a low-level rescue and autonomous replication of the wild-type AAV genome occurred in 293 but not in HeLa cells; (ii) mutations in the RBS resulted in augmented expression from the p5 promoter, leading to more efficient rescue and/or replication of the AAV genome in 293 but not in HeLa cells; (iii) little rescue and/or replication occurred from plasmids containing mutations in the YBS alone in the absence of coinfection with adenovirus; (iv) expression of the adenovirus E1A gene products was insufficient to mediate rescue and/or replication of the AAV genome in HeLa cells; (v) autonomously replicated AAV genomes in 293 cells were successfully encapsidated in mature progeny virions that were biologically active in secondary infection of HeLa cells in the presence of adenovirus; and (vi) stable transfection of recombinant AAV plasmids containing a gene for resistance to neomycin significantly affected stable integration only in 293 cells, presumably because rescue and autonomous replication of the AAV genome from these plasmids occurred in 293 cells but not in HeLa or KB cells. These data suggest that in the absence of adenovirus, the AAV Rep protein-RBS interaction plays a dominant role in down-regulating viral gene expression from the p5 promoter and that perturbation in this interaction is sufficient to confer autonomous replication competence to AAV in 293 cells.
Figures




Similar articles
-
Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions.J Virol. 1996 Mar;70(3):1668-77. doi: 10.1128/JVI.70.3.1668-1677.1996. J Virol. 1996. PMID: 8627687 Free PMC article.
-
Analysis of adeno-associated virus (AAV) wild-type and mutant Rep proteins for their abilities to negatively regulate AAV p5 and p19 mRNA levels.J Virol. 1994 May;68(5):2947-57. doi: 10.1128/JVI.68.5.2947-2957.1994. J Virol. 1994. PMID: 8151765 Free PMC article.
-
Negative regulation of the adeno-associated virus (AAV) P5 promoter involves both the P5 rep binding site and the consensus ATP-binding motif of the AAV Rep68 protein.J Virol. 1995 Nov;69(11):6787-96. doi: 10.1128/JVI.69.11.6787-6796.1995. J Virol. 1995. PMID: 7474090 Free PMC article.
-
High-level expression of adeno-associated virus (AAV) Rep78 or Rep68 protein is sufficient for infectious-particle formation by a rep-negative AAV mutant.J Virol. 1995 Nov;69(11):6880-5. doi: 10.1128/JVI.69.11.6880-6885.1995. J Virol. 1995. PMID: 7474103 Free PMC article.
-
The adeno-associated virus (AAV) Rep protein acts as both a repressor and an activator to regulate AAV transcription during a productive infection.J Virol. 1997 Feb;71(2):1079-88. doi: 10.1128/JVI.71.2.1079-1088.1997. J Virol. 1997. PMID: 8995628 Free PMC article.
Cited by
-
Adeno-Associated Virus Vector Mobilization, Risk Versus Reality.Hum Gene Ther. 2020 Oct;31(19-20):1054-1067. doi: 10.1089/hum.2020.118. Hum Gene Ther. 2020. PMID: 32829671 Free PMC article.
-
Identification of a Functionally Relevant Adeno-Associated Virus Rep68 Oligomeric Interface.J Virol. 2016 Jul 11;90(15):6612-6624. doi: 10.1128/JVI.00356-16. Print 2016 Aug 1. J Virol. 2016. PMID: 27170758 Free PMC article.
-
Reply to "D" matters in recombinant AAV packaging.Mol Ther. 2021 Sep 1;29(9):2628-2630. doi: 10.1016/j.ymthe.2021.08.012. Epub 2021 Aug 25. Mol Ther. 2021. PMID: 34437839 Free PMC article. No abstract available.
-
Productive replication of adeno-associated virus can occur in human papillomavirus type 16 (HPV-16) episome-containing keratinocytes and is augmented by the HPV-16 E2 protein.J Virol. 2000 Apr;74(8):3494-504. doi: 10.1128/jvi.74.8.3494-3504.2000. J Virol. 2000. PMID: 10729123 Free PMC article.
-
AAV vector transduction restriction and attenuated toxicity in hESCs via a rationally designed inverted terminal repeat.Nucleic Acids Res. 2025 Jan 24;53(3):gkaf013. doi: 10.1093/nar/gkaf013. Nucleic Acids Res. 2025. PMID: 39868534 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

