Role of interleukin-12 in primary influenza virus infection
- PMID: 9573248
- PMCID: PMC110027
- DOI: 10.1128/JVI.72.6.4825-4831.1998
Role of interleukin-12 in primary influenza virus infection
Abstract
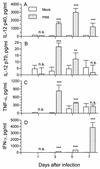
The effect of endogenous interleukin-12 (IL-12) on the influenza virus immune response in BALB/c mice was evaluated. Following primary influenza virus infection, IL-12 mRNA and protein are detected in the lung, with live virus being required for cytokine induction. Endogenous IL-12 contributes to early NK cell-dependent gamma interferon (IFN-gamma) production (days 3 and 5) but not late T-cell-dependent IFN-gamma secretion (day 7). IL-12 contributes to the inhibition of early virus replication but is not required for virus clearance. IL-12 also modestly contributes to the activation of cytotoxic T lymphocytes. Thus, in this model of experimental influenza virus infection, endogenous IL-12 contributes primarily to the early development and activation of the innate immune response.
Figures


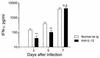
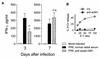


References
-
- Ada G L, Jones P D. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. - PubMed
-
- Barrette T, Inglis S C. Growth, purification and titration of influenza viruses. In: Mahy B W, editor. Virology, a practical approach. Oxford, United Kingdom: IRL Press; 1991. pp. 119–150.
-
- Bi Z, Quandt P, Komatsu T, Barna M, Reiss C S. IL-12 promotes enhanced recovery from vesicular stomatitis virus infection of the central nervous system. J Immunol. 1995;155:5684–5689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

