3'-Azido-3'-deoxythymidine (AZT) mediates cross-resistance to nucleoside analogs in the case of AZT-resistant human immunodeficiency virus type 1 variants
- PMID: 9573252
- PMCID: PMC110035
- DOI: 10.1128/JVI.72.6.4858-4865.1998
3'-Azido-3'-deoxythymidine (AZT) mediates cross-resistance to nucleoside analogs in the case of AZT-resistant human immunodeficiency virus type 1 variants
Abstract
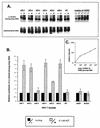
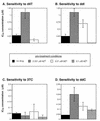
Difficulties in deciphering the mechanisms of 3'-azido-3'-deoxythymidine (AZT)-resistance by human immunodeficiency virus type 1 (HIV-1) variants are due in part to an inability to reconstitute resistance in vitro using AZT-resistant reverse transcriptases. We decided to characterize mechanisms of AZT resistance in tissue culture infections by studying the ability of drug-resistant viruses to synthesize viral DNA in the presence or absence of drug. Through use of PCR amplifications, we discovered an AZT-mediated stimulation of reverse transcription by AZT-resistant viruses carrying the M41L and T215Y mutations that can apparently override the inhibitory effects of AZT-5'-triphosphate. In addition, the presence of AZT also causes viruses containing the M41L and T215Y substitutions to have diminished sensitivity to other nucleoside analogs (i.e., ddC, ddI, and d4T). This AZT-mediated cross-resistance may help to explain the virological failure of treatment regimens that included ddI plus AZT or ddC plus AZT in situations in which the T215Y and/or M41L mutations were present (F. Brun-Vézinet, C. Boucher, C. Loveday, D. Descamps, V. Fauveau, J. Izopet, D. Jeffries, S. Kaye, C. Krzyanowski, A. Nunn, R. Schuurman, J. M. Seigneurin, C. Tamalet, R. Tedder, J. Weber, and G. J. Weverling, Lancet 350:983-990, 1997). Our results suggest that the use of AZT may be contraindicated in those patients for whom resistance to this compound (M41L and/or T215Y) has been demonstrated.
Figures

References
-
- Arts E J, Mak J, Kleiman L, Wainberg M A. DNA found in human immunodeficiency virus type 1 particles may not be required for infectivity. J Gen Virol. 1994;75:1605–1613. - PubMed
-
- Arts E J, Marois J P, Gu Z, Le Grice S F J, Wainberg M A. Effects of 3′-deoxynucleoside 5′-triphosphate concentrations on chain termination by nucleoside analogs during human immunodeficiency virus type-1 reverse transcription of minus-strand strong-stop DNA. J Virol. 1996;70:712–720. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

