Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family
- PMID: 9573291
- PMCID: PMC110095
- DOI: 10.1128/JVI.72.6.5189-5197.1998
Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family
Erratum in
-
Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family.J Virol. 1998 Oct;72(10):8461. J Virol. 1998. PMID: 9766974 Free PMC article. No abstract available.
Abstract
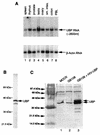
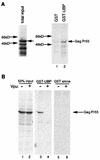
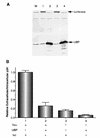
Viral protein U (Vpu) is a protein encoded by human immunodeficiency virus type 1 (HIV-1) that promotes the degradation of the virus receptor, CD4, and enhances the release of virus particles from cells. We isolated a cDNA that encodes a novel cellular protein that interacts with Vpu in vitro, in vivo, and in yeast cells. This Vpu-binding protein (UBP) has a molecular mass of 41 kDa and is expressed ubiquitously in human tissues at the RNA level. UBP is a novel member of the tetratricopeptide repeat (TPR) protein family containing four copies of the 34-amino-acid TPR motif. Other proteins that contain TPR motifs include members of the immunophilin superfamily, organelle-targeting proteins, and a protein phosphatase. UBP also interacts directly with HIV-1 Gag protein, the principal structural component of the viral capsid. However, when Vpu and Gag are coexpressed, stable interaction between UBP and Gag is diminished. Furthermore, overexpression of UBP in virus-producing cells resulted in a significant reduction in HIV-1 virion release. Taken together, these data indicate that UBP plays a role in Vpu-mediated enhancement of particle release.
Figures




References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

