Immunoglobulin A-specific capture enzyme-linked immunosorbent assay for diagnosis of dengue fever
- PMID: 9574674
- PMCID: PMC104797
- DOI: 10.1128/JCM.36.5.1189-1192.1998
Immunoglobulin A-specific capture enzyme-linked immunosorbent assay for diagnosis of dengue fever
Abstract
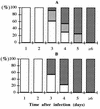
Dengue fever (DF) is usually diagnosed by testing for dengue virus immunoglobulin M (IgM) by a capture enzyme-linked immunosorbent assay (ELISA) (MAC-ELISA). However, IgM can last for months, and its presence might reflect a previous infection. We have tested the use of anti-dengue virus IgA capture ELISA (AAC-ELISA) for the diagnosis of DF by comparing the results of MAC-ELISAs and AAC-ELISAs for 178 serum samples taken from patients with confirmed cases of DF. IgM appears more rapidly (mean delay of positivity, 3.8 days after the onset of DF) than IgA (4.6 days) but lasts longer; the peak IgA titer is obtained on day 8. The specificity and the positive predictive value of AAC-ELISA are 100%; its sensitivity and negative predictive value (NPV) are also 100% between days 6 and 25 after the onset of DF, but they decrease drastically when data for tests conducted with specimens from the first days of infection are included, because the IgA titers, like the IgM titers, have not yet risen. AAC-ELISA is a simple method that can be performed together with MAC-ELISA and that can help in interpreting DF serology.
Figures

References
-
- Boisier P, Morvan J M, Laventure S, Charrier N, Martin E, Ouledi A, Roux J. Epidémie de dengue 1 sur l’île de la Grande Comore (République Fédérale Islamique des Comores). Mars-mai 1993. Ann Soc Belge Med Trop. 1994;74:217–229. - PubMed
-
- Bundo K, Igarashi A. Antibody-capture ELISA for detection of immunoglobulin M in sera from Japanese encephalitis and dengue hemorragic fever patients. J Virol Methods. 1985;11:15–22. - PubMed
-
- Cardosa M J, Zuraini I. Comparison of an IgM capture ELISA with a dot enzyme immunoassay for laboratory diagnosis of dengue virus infections. Southeast Asian J Trop Med Public Health. 1991;22:337–340. - PubMed
-
- Chen W J, Hwang K P, Fang A H. Detection of IgM antibodies from cerebrospinal fluid and sera of dengue fever patients. Southeast Asian J Trop Med Public Health. 1991;22:659–663. - PubMed
-
- Chow L, Hsu S T. MAC-ELISA for the detection of IgM antibodies to dengue type 1 virus. Chin J Microbiol Immunol (Taiwan) 1989;22:278–285. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

