Ultrastructure, immunofluorescence, western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum and urine samples and duodenal aspirates of five patients with AIDS
- PMID: 9574677
- PMCID: PMC104800
- DOI: 10.1128/JCM.36.5.1201-1208.1998
Ultrastructure, immunofluorescence, western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum and urine samples and duodenal aspirates of five patients with AIDS
Abstract
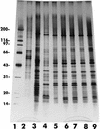
Microsporidia are ancient, intracellular, eukaryotic protozoan parasites that form spores and that lack mitochondria. Currently, as many as eight species included under six genera are known to infect humans, mostly patients with AIDS. Among these, Enterocytozoon bieneusi, the agent of gastrointestinal (GI) disease, is the most frequently identified microsporidian in clinical laboratories in the United States. Encephalitozoon (Septata) intestinalis, the agent that causes a disseminated infection including infection of the GI tract, is the second most frequently identified microsporidian parasite. In spite of this, not many isolates of E. intestinalis have been established in culture. We describe here the continuous cultivation of eight isolates of E. intestinalis obtained from different samples including the urine, sputum, and duodenal aspirate or biopsy specimens from five AIDS patients originating from California, Colorado, and Georgia. The specific identification was made on the bases of ultrastructural, antigenic, and PCR analyses.
Figures





References
-
- Black S S, Steinhort L A, Bertucci D C, Rogers L B, Didier E S. Encephalitozoon hellem in budgerigars. Vet Pathol. 1997;34:189–198. - PubMed
-
- Bornay-Llinares, F., et al. Unpublished data.
-
- Cali A, Kotler D P, Orenstein J M. Septata intestinalis, n.g., n.sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Protozool. 1993;40:101–112. - PubMed
-
- Canning E U, Lom J, Dykova I. The microsporidia of vertebrates. New York, N.Y: Academic Press, Inc.; 1986.
-
- Coyle C M, Wittner M, Kotler D P, Noyer C, Orenstein J M, Tanowitz H B, Weiss L M. Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin Infect Dis. 1996;23:1002–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

