Nonperinatal nosocomial transmission of Candida albicans in a neonatal intensive care unit: prospective study
- PMID: 9574687
- PMCID: PMC104810
- DOI: 10.1128/JCM.36.5.1255-1259.1998
Nonperinatal nosocomial transmission of Candida albicans in a neonatal intensive care unit: prospective study
Abstract
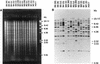
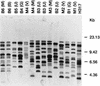
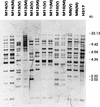
Nosocomial Candida albicans infections have become a major cause of morbidity and mortality in neonates in neonatal intensive care units (NICUs). To determine the possible modes of acquisition of C. albicans in hospitalized neonates, we conducted a prospective study at Grady Memorial Hospital, Atlanta, Ga. Clinical samples for fungal surveillance cultures were obtained at birth from infants (mouth, umbilicus, and groin) and their mothers (mouth and vagina) and were obtained from infants weekly until they were discharged. All infants were culture negative for C. albicans at birth. Six infants acquired C. albicans during their NICU stay. Thirty-four (53%) of 64 mothers were C. albicans positive (positive at the mouth, n = 26; positive at the vagina, n = 18; positive at both sites, n = 10) at the time of the infant's delivery. A total of 49 C. albicans isolates were analyzed by restriction endonuclease analysis and restriction fragment length polymorphism analysis by using genomic blots hybridized with the CARE-2 probe. Of the mothers positive for C. albicans, 3 of 10 were colonized with identical strains at two different body sites, whereas 7 of 10 harbored nonidentical strains at the two different body sites. Four of six infants who acquired C. albicans colonization in the NICU had C. albicans-positive mothers; specimens from all mother-infant pairs had different restriction endonuclease and CARE-2 hybridization profiles. One C. albicans-colonized infant developed candidemia; the colonizing and infecting strains had identical banding patterns. Our study indicates that nonperinatal nosocomial transmission of C. albicans is the predominant mode of acquisition by neonates in NICUs at this hospital; mothers may be colonized with multiple strains of C. albicans simultaneously; colonizing C. albicans strains can cause invasive disease in neonates; and molecular biology-based techniques are necessary to determine the epidemiologic relatedness of maternal and infant C. albicans isolates and to facilitate determination of the mode of transmission.
Figures
Similar articles
-
Molecular tracking of Candida albicans in a neonatal intensive care unit: long-term colonizations versus catheter-related infections.J Clin Microbiol. 1997 Dec;35(12):3032-6. doi: 10.1128/jcm.35.12.3032-3036.1997. J Clin Microbiol. 1997. PMID: 9399489 Free PMC article.
-
Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques.Pediatr Infect Dis J. 2008 Mar;27(3):231-5. doi: 10.1097/INF.0b013e31815bb69d. Pediatr Infect Dis J. 2008. PMID: 18277930
-
Possible transmission of Candida albicans on an intensive care unit: genotype and temporal cluster analyses.J Hosp Infect. 2013 Sep;85(1):60-5. doi: 10.1016/j.jhin.2013.06.002. Epub 2013 Aug 5. J Hosp Infect. 2013. PMID: 23927923
-
The use of molecular techniques for epidemiologic typing of Candida species.Curr Top Med Mycol. 1992;4:43-63. doi: 10.1007/978-1-4612-2762-5_2. Curr Top Med Mycol. 1992. PMID: 1732071 Review.
-
Candida albicans - Biology, molecular characterization, pathogenicity, and advances in diagnosis and control - An update.Microb Pathog. 2018 Apr;117:128-138. doi: 10.1016/j.micpath.2018.02.028. Epub 2018 Feb 16. Microb Pathog. 2018. PMID: 29454824 Review.
Cited by
-
Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm infants: a double-blind, placebo-controlled, randomized study.J Clin Microbiol. 2006 Nov;44(11):4025-31. doi: 10.1128/JCM.00767-06. Epub 2006 Sep 13. J Clin Microbiol. 2006. PMID: 16971641 Free PMC article. Clinical Trial.
-
Emerging Issues in Nosocomial Fungal Infections.Curr Infect Dis Rep. 1999 Oct;1(4):347-361. doi: 10.1007/s11908-999-0041-3. Curr Infect Dis Rep. 1999. PMID: 11095808
-
Invasive Candida Infections in Neonatal Intensive Care Units: Risk Factors and New Insights in Prevention.Pathogens. 2024 Aug 6;13(8):660. doi: 10.3390/pathogens13080660. Pathogens. 2024. PMID: 39204260 Free PMC article. Review.
-
A murine model for disseminated candidiasis in neonates.Pediatr Res. 2011 Mar;69(3):189-93. doi: 10.1203/PDR.0b013e318206fd3e. Pediatr Res. 2011. PMID: 21099449 Free PMC article.
-
Electrophoretic karyotyping of Candida albicans strains isolated from premature infants and hospital personnel in a neonatal intensive care unit.Folia Microbiol (Praha). 2001;46(5):453-7. doi: 10.1007/BF02814438. Folia Microbiol (Praha). 2001. PMID: 11899481
References
-
- Baley J E, Kliegman R M, Boxerbaum B, Fanaroff A A. Fungal colonization in the very-low-birth-weight infant. Pediatrics. 1986;78:225–232. - PubMed
-
- Baley J E, Kliegman R M, Fanaroff A A. Disseminated fungal infections in low-birth-weight infants: clinical manifestations and epidemiology. Pediatrics. 1984;73:144–152. - PubMed
-
- Beck-Sague C, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. - PubMed
-
- Bektas S, Goetze B, Speer C P. Decreased adherence, chemotaxis and phagocytic activities of neutrophils from preterm neonates. Acta Pediatr Scand. 1990;79:1031–1038. - PubMed
-
- Betremieux P, Chevrier S, Quindos G, Sullivan D, Polonelli L, Guiguen C. Use of DNA fingerprinting and biotyping methods to study a Candida albicans outbreak in a neonatal intensive care unit. Pediatr Infect Dis J. 1994;13:899–905. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

