Effect of nitric oxide on single skeletal muscle fibres from the mouse
- PMID: 9575305
- PMCID: PMC2230981
- DOI: 10.1111/j.1469-7793.1998.577bn.x
Effect of nitric oxide on single skeletal muscle fibres from the mouse
Abstract
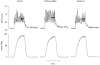
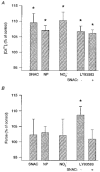
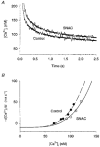
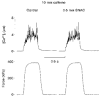
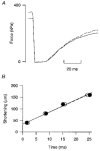
1. Single skeletal muscle fibres from a mouse foot muscle were used to investigate the effects of nitric oxide on contractile function. 2. We measured force production and myoplasmic free Ca2+ concentration ([Ca2+]i) in single fibres exposed to the nitric oxide donors S-nitroso-N-acetylcysteine (SNAC) and nitroprusside. 3. The nitric oxide donors reduced myofibrillar Ca2+ sensitivity, whereas [Ca2+]i transients were increased during submaximal tetani. Force was largely unchanged. SNAC did not change maximum shortening velocity, the rate of force redevelopment, or force production at saturating [Ca2+]i. 4. The guanylyl cyclase inhibitor LY83583 increased tetanic [Ca2+]i but had no effect on Ca2+ sensitivity. LY83583 did not prevent the decrease in myofibrillar Ca2+ sensitivity in response to SNAC. The oxidizer sodium nitrite increased tetanic [Ca2+]i and decreased myofibrillar Ca2+ sensitivity. 5. We conclude that under our experimental conditions nitric oxide impairs Ca2+ activation of the actin filaments which results in decreased myofibrillar Ca2+ sensitivity.
Figures
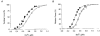
References
-
- Aghdasi B, Reid MB, Hamilton SL. Nitric oxide protects the skeletal muscle Ca2+ release channel from oxidation induced activation. Journal of Biological Chemistry. 1997;272:25462–25467. 10.1074/jbc.272.41.25462. - DOI - PubMed
-
- Aghdasi B, Zhang J-Z, Wu Y, Reid MB, Hamilton SL. Multiple classes of sulfhydryls modulate the skeletal muscle Ca2+ release channel. Journal of Biological Chemistry. 1997;272:3739–3748. 10.1074/jbc.272.6.3739. - DOI - PubMed
-
- Arnelle DR, Stamler JS. NO+, NO•, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Archives of Biochemistry and Biophysics. 1995;318:279–285. 10.1006/abbi.1995.1231. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

