Purification and characterization of a low-molecular-weight phospholipase A2 from developing seeds of elm
- PMID: 9576789
- PMCID: PMC35004
- DOI: 10.1104/pp.117.1.197
Purification and characterization of a low-molecular-weight phospholipase A2 from developing seeds of elm
Abstract
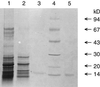
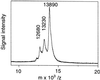
Phospholipase A2 (PLA2) was purified about 180,000 times compared with the starting soluble-protein extract from developing elm (Ulmus glabra) seeds. On sodium dodecyl sulfate-polyacrylamide gel electrophoresis the purified fraction showed a single protein band with a mobility that corresponded to 15 kD, from which activity could be recovered. When analyzed by matrix-assisted laser-desorption ionization-time-of-flight mass spectrometry, the enzyme had a deduced mass of 13,900 D. A 53-amino acid-long N-terminal sequence was determined and aligned with other sequences, giving 62% identity to the deduced amino acid sequence of some rice (Oryza sativa) expressed sequence tag clones. The purified enzyme had an alkaline pH optimum and required Ca2+ for activity. It was unusually stable with regard to heat, acidity, and organic solvents but was sensitive to disulfide bond-reducing agents. The enzyme is a true PLA2, neither hydrolyzing the sn-1 position of phosphatidylcholine nor having any activity toward lysophosphatidylcholine or diacylglycerol. The biochemical data and amino acid sequence alignments indicate that the enzyme is related to the well-characterized family of animal secretory PLA2s and, to our knowledge, is the first plant enzyme of this type to be described.
Figures



References
-
- Bafor M, Smith MA, Jonsson L, Stobart AK, Stymne S. Biosynthesis of vernoleate (cis-12-epoxyoctadeca-cis-9-enoate) in microsomal preparations from developing endosperm of Euphorbia lagascae. Arch Biochem Biophys. 1993;303:145–151. - PubMed
-
- Banas A, Johansson I, Stymne S. Plant microsomal phospholipases exhibit preference for phosphatidylcholine with oxygenated acyl groups. Plant Sci. 1992;84:137–144.
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

