Trends in odor intensity for human and electronic noses: relative roles of odorant vapor pressure vs. molecularly specific odorant binding
- PMID: 9576901
- PMCID: PMC20396
- DOI: 10.1073/pnas.95.10.5442
Trends in odor intensity for human and electronic noses: relative roles of odorant vapor pressure vs. molecularly specific odorant binding
Abstract
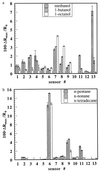
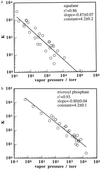
Response data were collected for a carbon black-polymer composite electronic nose array during exposure to homologous series of alkanes and alcohols. The mean response intensity of the electronic nose detectors and the response intensity of the most strongly driven set of electronic nose detectors were essentially constant for members of a chemically homologous odorant series when the concentration of each odorant in the gas phase was maintained at a constant fraction of the odorant's vapor pressure. A similar trend is observed in human odor detection threshold values for these same homologous series of odorants. Because the thermodynamic activity of an odorant at equilibrium in a sorbent phase is equal to the partial pressure of the odorant in the gas phase divided by the vapor pressure of the odorant and because the activity coefficients are similar within these homologous series of odorants for sorption of the vapors into specific polymer films, the data imply that the trends in detector response can be understood based on the thermodynamic tendency to establish a relatively constant concentration of sorbed odorant into each of the polymeric films of the electronic nose at a constant fraction of the odorant's vapor pressure. Similarly, the data are consistent with the hypothesis that the odor detection thresholds observed in human psychophysical experiments for the odorants studied herein are driven predominantly by the similarity in odorant concentrations sorbed into the olfactory epithelium at a constant fraction of the odorant's vapor pressure.
Figures



References
-
- Devos M, Patte F, Rouault J, Laffort P, Van Gemert L J. Standardized Human Olfactory Thresholds. New York: Oxford Univ. Press; 1990.
-
- Ohloff G. Scent and Fragrances, the Fashion of Odors and Their Chemical Perspectives. New York: Springer; 1994.
-
- Amoore J E. Molecular Basis of Odour. Springfield, IL: Thomas; 1970.
-
- Dravnieks A. Flavor Quality: Objective Measurement. Washington, DC: Am. Chem. Soc.; 1977. pp. 11–28.
-
- Edwards P A, Jurs P C. Chem Senses. 1989;14:281–291.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

