Development of normal mice from metaphase I oocytes fertilized with primary spermatocytes
- PMID: 9576931
- PMCID: PMC20426
- DOI: 10.1073/pnas.95.10.5611
Development of normal mice from metaphase I oocytes fertilized with primary spermatocytes
Abstract
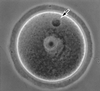
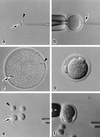
Primary spermatocytes are the male germ cells before meiosis I. To examine whether these 4n diploid cells are genetically competent to fertilize oocytes and support full embryo development, we introduced the nuclei of pachytene/diplotene spermatocytes into oocytes that were arrested in prophase I (germinal vesicle stage), metaphase I, or metaphase II (Met II). Both the paternal and maternal chromosomes then were allowed to undergo meiosis synchronously until Met II. In the first and second groups, the paternal and maternal chromosomes had intermingled to form a large Met II plate, which was then transferred into a fresh enucleated Met II oocyte. In the third group, the paternal Met II chromosomes were obtained by transferring spermatocyte nuclei into Met II oocytes twice. After activation of the Met II oocytes that were produced, those microfertilized at metaphase I showed the best developmental ability in vitro, and three of these embryos developed into full-term offspring after embryo transfer. Two pups (one male and one female) were proven to be fertile. This finding provides direct evidence that the nuclei of male germ cells acquire the ability to fertilize oocytes before the first meiotic division.
Figures




References
-
- Kimura Y, Yanagimachi R. Development. 1995;121:2397–2405. - PubMed
-
- Sofikitis N V, Miyagawa I, Agapitos E, Pasyianos P, Toda T, Hellstrom W J G, Kawamura H. J Assist Reprod Genet. 1994;11:335–341. - PubMed
-
- Tesarik J, Mendoza C, Testart J. N Engl J Med. 1995;333:525. - PubMed
-
- Kimura Y, Yanagimachi R. Biol Reprod. 1995;53:855–862. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

