Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells
- PMID: 9576950
- PMCID: PMC20445
- DOI: 10.1073/pnas.95.10.5718
Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells
Abstract
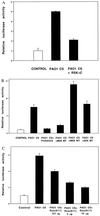
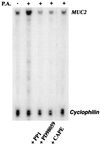
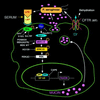
Cystic fibrosis (CF) is an autosomal recessive disorder, the most common lethal genetic disease in Caucasians. Respiratory disease is the major cause of morbidity and mortality. Indeed, 95% of CF patients die of respiratory failure. Pseudomonas aeruginosa, an opportunistic pathogen, chronically infects the lungs of over 85% of CF patients. It is ineradicable by antibiotics and responsible for airway mucus overproduction that contributes to airway obstruction and death. The molecular mechanisms underlying this pathology are unknown. Here we show that P. aeruginosa activates a c-Src-Ras-MEK1/2-MAPK-pp90rsk signaling pathway that leads to activation of nuclear factor NF-kappaB (p65/p50). Activated NF-kappaB binds to a kappaB site in the 5'-flanking region of the MUC2 gene and activates MUC2 mucin transcription. These studies bring new insight into bacterial-epithelial interactions and more specifically into the molecular pathogenesis of cystic fibrosis. Understanding these signaling and gene regulatory mechanisms opens up new therapeutic targets for cystic fibrosis.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

