Genetic induction of immune tolerance to human clotting factor VIII in a mouse model for hemophilia A
- PMID: 9576953
- PMCID: PMC20448
- DOI: 10.1073/pnas.95.10.5734
Genetic induction of immune tolerance to human clotting factor VIII in a mouse model for hemophilia A
Abstract
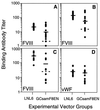
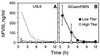
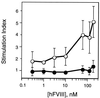
Patients with severe coagulation factor VIII deficiency require frequent infusions of human factor VIII (hFVIII) concentrates to treat life-threatening hemorrhages. Because these patients are immunologically hFVIII-naive, a significant treatment complication is the development of inhibitors or circulating alloantibodies against hFVIII, which bind the replaced glycoprotein, increase its plasma clearance, and inhibit its activity, preventing subsequent treatments from having a therapeutic effect. A genetic approach toward the induction of immunologic unresponsiveness to hFVIII has the conceptual advantage of a long-term, stable elimination of undesired immune responses against hFVIII. Here, we report that in a factor VIII (FVIII)-deficient mouse model for severe hemophilia A, genetic modification of donor bone marrow cells with a retroviral vector encoding hFVIII, and transplant to hemophiliac mouse recipients, results in the induction of immune tolerance to FVIII in 50% of treated animals after immunization with hFVIII, despite the fact that hFVIII protein or activity is undetectable. In tolerized animals, the titers of anti-hFVIII binding antibodies and of hFVIII inhibitor antibodies were significantly reduced, and there was evidence for hFVIII unresponsiveness in CD4(+) T cells. Importantly, the plasma clearance of hFVIII was significantly decreased in tolerized animals and was not significantly different from that seen in a FVIII-naive hemophiliac mouse. This model system will prove useful for the evaluation of genetic therapies for hFVIII immunomodulation and bring genetic therapies for hFVIII tolerance closer to clinical application for patients with hemophilia A.
Figures

References
-
- Antonarakis S E, Youssoufian H, Kazazian H H., Jr Mol Biol Med. 1987;4:81–94. - PubMed
-
- Wion K L, Kelly D, Summerfield J A, Tuddenham E G D, Lawn R M. Nature (London) 1985;317:726–728. - PubMed
-
- Vehar G A, Keyt B, Eaton D, Rodriguez H, O’Brien D P, Rotblat F, Oppermann H, Keck R, Lawn R M, Capon D J. Nature (London) 1984;312:337–342. - PubMed
-
- Brettler D. Baillieres Clin Haematol. 1996;9:319–329. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

